Micro-algae come of age as a platform for recombinant protein production
- PMID: 20556634
- PMCID: PMC2941057
- DOI: 10.1007/s10529-010-0326-5
Micro-algae come of age as a platform for recombinant protein production
Abstract
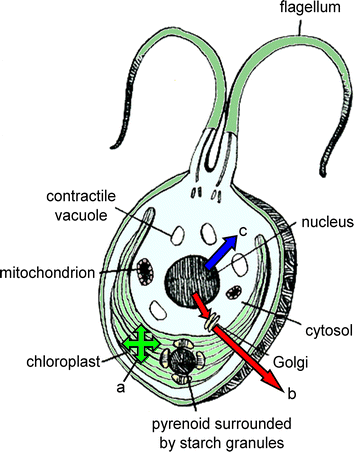
A complete set of genetic tools is still being developed for the micro-alga Chlamydomonas reinhardtii. Yet even with this incomplete set, this photosynthetic single-celled plant has demonstrated significant promise as a platform for recombinant protein expression. In recent years, techniques have been developed that allow for robust expression of genes from both the nuclear and plastid genome. With these advances, many research groups have examined the pliability of this and other micro-algae as biological machines capable of producing recombinant peptides and proteins. This review describes recent successes in recombinant protein production in Chlamydomonas, including production of complex mammalian therapeutic proteins and monoclonal antibodies at levels sufficient for production at economic parity with existing production platforms. These advances have also shed light on the details of algal protein production at the molecular level, and provide insight into the next steps for optimizing micro-algae as a useful platform for the production of therapeutic and industrially relevant recombinant proteins.
Figures
Similar articles
-
Efficient recombinant protein production and secretion from nuclear transgenes in Chlamydomonas reinhardtii.J Biotechnol. 2013 Aug 20;167(2):101-10. doi: 10.1016/j.jbiotec.2012.10.010. Epub 2012 Oct 22. J Biotechnol. 2013. PMID: 23099045
-
Production of Recombinant Proteins in the Chloroplast of the Green Alga Chlamydomonas reinhardtii.Methods Mol Biol. 2016;1385:69-85. doi: 10.1007/978-1-4939-3289-4_5. Methods Mol Biol. 2016. PMID: 26614282
-
Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses.Photosynth Res. 2015 Mar;123(3):227-39. doi: 10.1007/s11120-014-9994-7. Epub 2014 Mar 22. Photosynth Res. 2015. PMID: 24659086 Review.
-
The microalga Chlamydomonas reinhardtii as a platform for the production of human protein therapeutics.Bioeng Bugs. 2011 Jan-Feb;2(1):50-4. doi: 10.4161/bbug.2.1.13423. Bioeng Bugs. 2011. PMID: 21636988 Free PMC article.
-
Chlamydomonas reinhardtii: a protein expression system for pharmaceutical and biotechnological proteins.Mol Biotechnol. 2006 Oct;34(2):213-23. doi: 10.1385/MB:34:2:213. Mol Biotechnol. 2006. PMID: 17172667 Review.
Cited by
-
Microalgal Cell Biofactory-Therapeutic, Nutraceutical and Functional Food Applications.Plants (Basel). 2021 Apr 21;10(5):836. doi: 10.3390/plants10050836. Plants (Basel). 2021. PMID: 33919450 Free PMC article. Review.
-
Can algae contribute to the war with Covid-19?Bioengineered. 2021 Dec;12(1):1226-1237. doi: 10.1080/21655979.2021.1910432. Bioengineered. 2021. PMID: 33858291 Free PMC article. Review.
-
Unassembled cell wall proteins form aggregates in the extracellular space of Chlamydomonas reinhardtii strain UVM4.Appl Microbiol Biotechnol. 2022 Jun;106(11):4145-4156. doi: 10.1007/s00253-022-11960-9. Epub 2022 May 23. Appl Microbiol Biotechnol. 2022. PMID: 35599258 Free PMC article.
-
An efficient protocol for the Agrobacterium-mediated genetic transformation of microalga Chlamydomonas reinhardtii.Mol Biotechnol. 2014 Jun;56(6):507-15. doi: 10.1007/s12033-013-9720-2. Mol Biotechnol. 2014. PMID: 24198218
-
Physiological and Ecological Aspects of Chlorella sorokiniana (Trebouxiophyceae) Under Photoautotrophic and Mixotrophic Conditions.Microb Ecol. 2018 Oct;76(3):791-800. doi: 10.1007/s00248-018-1170-8. Epub 2018 Mar 8. Microb Ecol. 2018. PMID: 29520451
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

