Concurrent chemoradiotherapy in non-small cell lung cancer
- PMID: 20556756
- PMCID: PMC12584098
- DOI: 10.1002/14651858.CD002140.pub3
Concurrent chemoradiotherapy in non-small cell lung cancer
Abstract
Background: This is an updated version of the original review published in Issue 4, 2004. The use of concurrent chemotherapy and radiotherapy in non-small cell lung cancer (NSCLC) might be seen as a way of increasing the effectiveness of radical radiotherapy at the same time as reducing the risks of metastatic disease.
Objectives: To determine the effectiveness of concurrent chemoradiotherapy as compared to radiotherapy alone with regard to overall survival, tumour control and treatment-related morbidity. To determine the effectiveness of concurrent versus sequential chemoradiotherapy.
Search strategy: For this update we ran a new search in October 2009, using a search strategy adapted from the design in the original review. We searched: CENTRAL (accessed through The Cochrane Library, 2009, Issue 4), MEDLINE (accessed through PubMed), and EMBASE (accessed through Ovid).
Selection criteria: Randomised trials of patients with stage I-III NSCLC undergoing radical radiotherapy and randomised to receive concurrent chemoradiotherapy versus radiotherapy alone, or concurrent versus sequential chemoradiotherapy.
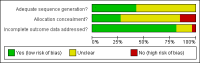
Data collection and analysis: Study selection, data extraction and assessment of risk of bias was performed independently by two authors. Pooled hazard ratios and relative risks were calculated according to a random-effects model.
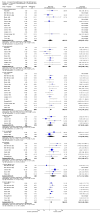
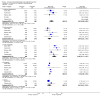
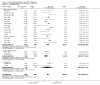
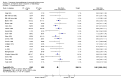
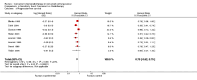
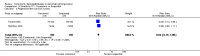
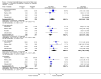
Main results: Nineteen randomised studies (2728 participants) of concurrent chemoradiotherapy versus radiotherapy alone were included. Chemoradiotherapy significantly reduced overall risk of death (HR 0.71, 95% CI 0.64 to 0.80; I(2) 0%; 1607 participants) and overall progression-free survival at any site (HR 0.69, 95% CI 0.58 to 0.81; I(2) 45%; 1145 participants). Incidence of acute oesophagitis, neutropenia and anaemia were significantly increased with concurrent chemoradiation. Six trials (1024 patients) of concurrent versus sequential chemoradiation were included. A significant benefit of concurrent treatment was shown in overall survival (HR 0.74, 95% CI 0.62 to 0.89; I(2) 0%; 702 participants). This represented a 10% absolute survival benefit at 2 years. More treatment-related deaths (4% vs 2%) were reported in the concurrent arm without statistical significance (RR 2.02, 95% CI 0.90 to 4.52; I(2) 0%; 950 participants). There was increased severe oesophagitis with concurrent treatment (RR 4.96, 95%CI 2.17 to 11.37; I(2) 66%; 947 participants).
Authors' conclusions: This update of the review published in 2004 incorporates additional trials and more mature data. It demonstrates the benefit of concurrent chemoradiation over radiotherapy alone or sequential chemoradiotherapy. Patient selection is an important consideration in view of the added toxicity of concurrent treatment. Uncertainty remains as to how far this is purely due to a radiosensitising effect and whether similar benefits could be achieved by using modern radiotherapy techniques and more dose intensive accelerated and/ or hyperfractionated radiotherapy regimens.
Conflict of interest statement
None known.
Figures





































Update of
-
Concurrent chemoradiotherapy in non-small cell lung cancer.Cochrane Database Syst Rev. 2004 Oct 18;(4):CD002140. doi: 10.1002/14651858.CD002140.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2010 Jun 16;(6):CD002140. doi: 10.1002/14651858.CD002140.pub3. PMID: 15495029 Updated.
References
References to studies included in this review
Atagi 2005 {published data only}
-
- Atagi S, Kawahara M, Tamura T, Noda K, Watanabe K, Yokoyama A, et al. Standard thoracic radiotherapy with or without concurrent daily low‐dose carboplatin in elderly patients with locally advanced non‐small cell lung cancer: A phase III trial of the Japan Clinical Oncology Group (JCOG9812). Japanese Journal of Clinical Oncology 2005;35(4):195‐201. - PubMed
Ball 1999 once daily {published data only}
-
- Ball D, Bishop J, Smith J, O'Brien P, Davis S, Ryan G, et al. A randomised phase II study of accelerated or standard fraction radiotherapy with or without concurrent carboplatin in inoperable non‐small cell lung cancer: final report of an Australian multi‐centre trial. Radiotherapy and Oncology 1999;52(2):129‐36. - PubMed
Ball 1999 twice daily {published data only}
-
- Ball D, Bishop J, Smith J, O'Brien P, Davis S, Ryan G, et al. A randomised phase II study of accelerated or standard fraction radiotherapy with or without concurrent carboplatin in inoperable non‐small cell lung cancer: final report of an Australian multi‐centre trial. Radiotherapy and Oncology 1999;52(2):129‐36. - PubMed
Blanke 1995 {published data only}
-
- Ansari R, Tokars R, Fisher W, Pennington K, Mantravadi R, O'Connor T, et al. A phase III study of thoracic irradiation with or without concomitant cisplatin in locoregional unresectable non small cell lung cancer: a Hoosier Oncology Group Protocol. Proceedings of the American Society of Clinical Oncology 1991;10:241. - PubMed
-
- Blanke C, Ansari R, Mantravadi R, Gonin R, Tokars R, Fisher W, et al. Phase III trial of thoracic irradiation with or without cisplatin for locally advanced unresectable non‐small‐cell lung cancer: a Hoosier Oncology Group Protocol. Journal of Clinical Oncology 1995;13(6):1425‐9. - PubMed
Bonner 1998 {published data only}
-
- Bonner JA, McGinnis WL, Stella PJ, Marschke FR, Sloan JA, Shaw EG, et al. The possible advantage of hyperfractionated thoracic radiotherapy in the treatment of locally advanced non‐small lung carcinoma. Cancer 1996;82(6):1037‐48. - PubMed
Cakir 2004 {published data only}
-
- Cakir S, Egehan I. A randomised clinical trial of radiotherapy plus cisplatin versus radiotherapy alone in stage III non‐small cell lung cancer. Lung Cancer 2004;43:309‐16. - PubMed
Clamon 1999 {published data only}
-
- Clamon G, Herndon J, Cooper R, Chang AY, Rosenman J, Green MR. Radiosensitization with carboplatin for patients with unresectable stage III non‐small‐cell lung cancer: a phase III trial of the Cancer and Leukaemia Group B and the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 1999;17(1):4‐11. - PubMed
Curran 2003 {published and unpublished data}
-
- Curran WJ, Scott CB, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Long‐term benefit is observed in a phase III comparison of sequential vs concurrent chemoradiation for patients with unresected stage III NSCLC: RTOG 9410. Proceedings of the American Society of Clinical Oncology 2003;22:621.
Fournel 2001 {published data only}
-
- Fournel P, Perol M, Robinet G, Thomas P, Souquet JP, Lena H, et al. A randomized phase III trial of sequential chemo‐radiotherapy versus concurrent chemo‐radiotherapy in locally advanced non small cell lung cancer (NSCLC) (GLOT‐GFPC NPC 95‐01 study) [abstract]. Proceedings of the American Society of Clinical Oncology 2001;20:312.
-
- Fournel P, Robinet G, Thomas P, Souquet PJ, Lena H, Vergnenegre A, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non‐small‐cell lung cancer: Groupe Lyon‐Saint‐Etienne d'Oncologie Thoracique‐Groupe Francais de Pneumo‐Cancerologie NPC 95‐01 Study. Journal of Clinical Oncology 2005;23(25):5910‐7. - PubMed
-
- Vergnenegre A, Combescure C, Fournel P, Bayle S, Gimenez C, Souquet PJ, et al. Cost‐minimization analysis of a phase III trial comparing concurrent versus sequential radiochemotherapy for locally advanced non‐small‐cell lung cancer (GFPC‐GLOT 95‐01). Annals of Oncology 2006;17(8):1269‐74. - PubMed
Gouda 2006 {published data only}
-
- Gouda YS, Kohail HM, Eldeeb NA, Omar AM, El‐Geneidy MM, Elkerm YM. Randomized study of concurrent carboplatin, paclitaxel, and radiotherapy with or without prior induction chemotherapy in patients with locally advanced non‐small cell lung cancer. Journal of the Egyptian National Cancer Institute 2006;18(1):73‐81. - PubMed
Groen 1999 {published and unpublished data}
-
- Groen HJ, Leest AH, Fokkema E, Timmer PR, Nossent GD, Smit WJ, et al. Continuously infused carboplatin used as radiosensitizer in locally unresectable non‐small‐cell lung cancer: a multicenter phase III study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2004;15(3):427‐32. - PubMed
-
- Groen HJM, Leest AHW, Snoek WJ, Nossent GD, Oosterhuis B, Nabers H, et al. Phase III study of continuous infusion carboplatin over 6 weeks with radiation versus radiation alone in stage III non‐small cell lung cancer [abstract]. Proceedings of the American Society of Clinical Oncology 1999;18:466.
Huber 2003 {published and unpublished data}
-
- Huber RM, Flentje M, Schmidt M, Pöllinger B, Gosse H, Willner J, et al. Simultaneous chemoradiotherapy compared with radiotherapy alone after induction chemotherapy in inoperable stage IIIA or IIIB non‐small‐cell lung cancer: study CTRT99/97 by the Bronchial Carcinoma Therapy Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2006;24(27):4397‐404. - PubMed
-
- Huber RM, Schmidt M, Flentje M, Poellinger B, Gosse H, Willner J, et al for the BROCAT group. Induction chemotherapy and following simultaneous radio/chemotherapy versus induction chemotherapy and radiotherapy alone in inoperable NSCLC (stage IIIA/IIIB). Proceedings of the American Society of Clinical Oncology 2003;22:622. - PubMed
Jeremic 1995 {published data only}
-
- Jeremic B, Shibamoto Y, Acimovic L, Djuric L. Randomized trial of hyperfractionated radiation therapy with or without concurrent chemotherapy for stage III non‐small‐cell lung cancer. Journal of Clinical Oncology 1995;13(2):452‐8. - PubMed
Jeremic 1996 {published data only}
-
- Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Hyperfractionated radiation therapy with or without concurrent low‐dose daily carboplatin / etoposide for stage III non‐small‐cell lung cancer: a randomized study. Journal of Clinical Oncology 1996;14(4):1065‐70. - PubMed
Landgren 1974 {published data only}
-
- Landgren RC, Hussey DH, Barkley HT, Samuels ML. Split‐course irradiation compared to split‐course irradiation plus hydroxyurea in inoperable bronchogenic carcinoma ‐ a randomized study of 53 patients. Cancer 1974;34:1598‐1601. - PubMed
Li 2008 {published data only}
-
- Li GZ, Zhang SH, Zhang Z, Song WG, Wang RL, Fu ZZ. The clinical comparative study on hydroxycomptothecin combined with cocurrent hyperfractionated radiotherapy for unresectable stage III non‐small cell lung cancer. Tumor 2008;28(2):156‐8.
Lu 2005 {published data only}
-
- Lu C, Liu J, Wang J, Wang C, Guo J, Pan F, et al. Analysis of concurrent chemoradiotherapy in patients with stage III non‐small cell lung cancer after two cycles of induction chemotherapy. Chinese Journal of Lung Cancer 2005;8(1):48‐50. - PubMed
Manegold 2003 {published data only}
-
- Manegold C, Debus J, Buchholz E, Scagliotti GV, Ricardi U, Cardenal F, et al. A phase II randomized study comparing docetaxel/cisplatin induction therapy followed by thoracic radiotherapy with or without weekly docetaxel in unresectable stage IIIA‐IIIB non‐small cell lung cancer. European Journal of Cancer 2003;1 Suppl(5):248‐9.
-
- Ramlau R, Scagliotti GV, Manegold C, Buchholz E, Szczesna A, Cardenal F, et al. Radiotherapy and concurrent weekly docetaxel following cisplatin‐docetaxel induction chemotherapy in unresectable stage IIIA‐B non‐small‐cell lung cancer: a randomized phase II trial. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2002;13(suppl 5):134.
-
- Scagliotti GV, Manegold C, Bucholtz E, Zierhut D, Szczesna A, Kaczmarek Z, et al. Randomized phase II study evaluating the feasibility of thoracic radiotherapy with or without weekly docetaxel (Taxotere) following induction chemotherapy with cisplatin (DDP) and docetaxel in unresectable stage IIIA/B non‐small cell lung cancer. Proceedings of the American Society of Clinical Oncology 2002;21:320a (abstr 1279).
Rao 2007 {published data only}
-
- Rao CZ. Study of concurrent versus sequential chemoradiotherapy with vinorelbine and cisplatin is stage III non‐small cell lung cancer. Chinese Journal of Cancer Prevention and Treatment 2007;14(12):942‐3.
Reinfuss 2005 {published data only}
-
- Reinfuss M, Skolyszewski J, Kowalska T, Glinski B, Dymek P, Walasek T, et al. Evaluation of efficacy of combined chemoradiotherapy in locoregional advanced, inoperable Non‐Small Cell Lung Cancer (clinical randomized trial). Nowotwory 2005;55(3):200‐6.
Schaake‐Koning 1992 {published data only}
-
- Schaake‐Koning C, Bogert W, Dalesio O, Festen J, Hoogenhout J, Houtte P, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non‐small‐cell lung cancer. The New England Journal of Medicine 1992;326(8):524‐30. - PubMed
Soresi 1988 {published data only}
-
- Soresi E, Clerici M, Grilli R, Borghini U, Zucali R, Leoni M, et al. A randomized clinical trial comparing radiation therapy v radiation therapy plus cis‐dichlorodiammine platinum (II) in the treatment of locally advanced non‐small cell lung cancer. Seminars in Oncology 1988;15 Suppl 7(6):20‐5. - PubMed
Trovo 1992 {published data only}
-
- Trovo MG, Minatel E, Franchin G, Boccieri MG, Nascimben O, Bolzicco G, et al. Radiotherapy versus radiotherapy enhanced by cisplatin in stage III non‐small cell lung cancer. International Journal of Radiation Oncology, Biology, Physics 1992;24(1):11‐5. - PubMed
Wu 2006 {published data only}
-
- Wu H, Zhou D, Wu Q, Cai Y, Liu Y, Jiang J. [Clinical trial of concurrent low‐dose chemotherapy plus radiation vs sequential chemoradiotherapy for unresectable stage III non‐small cell lung cancer]. Chinese Journal of Lung Cancer 2006;9(3):283‐5. - PubMed
Yadav 2005 {published data only}
-
- Yadav B S, Ghoshal S, Sharma SC, Behera D, Kapoor V. Radiotherapy versus weekly concomitant cisplatin in locally advanced non‐small cell lung cancer ‐ a pilot study. Bulletin, Postgraduate Institute of Medical Education & Research, Chandigarh 2005;39(2):75‐80.
Zatloukal 2003 {published data only}
-
- Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non‐small cell lung cancer: A randomized study. Lung Cancer 2004;46(1):87‐98. - PubMed
-
- Zatloukal P, Petruzelka L, Zemanova M, Havel L, Pecen L, Judas L. Concurrent versus sequential radiochemotherapy with vinorelbine plus cisplatin (V‐P) in locally advanced non‐small cell lung cancer. A randomized phase II study ‐ long‐term follow‐up data. Lung Cancer 2003;41 Suppl(2):76.
-
- Zatloukal P, Petruzelka L, Zemanova M, Havel L, Pecen L, Krepela E, et al. Concurrent versus sequential radiochemotherapy with vinorelbine plus cisplatin (V‐P) in locally advanced non‐small cell lung cancer. A randomized phase II study [abstract]. Proceedings of the American Society of Clinical Oncology 2002;21:290.
References to studies excluded from this review
Ball 1997 {published data only}
Belderbos 2007 {published data only}
-
- Belderbos J, Uitterhoeve L, Zandwijk N, Belderbos H, Rodrigus P, Vaart P, et al. Randomised trial of sequential versus concurrent chemo‐radiotherapy in patients with inoperable non‐small cell lung cancer (EORTC 08972‐22973). European Journal of Cancer 2007;43(1):114‐21. - PubMed
Chan 1976 {published data only}
-
- Chan PYM, Byfield JE, Kagan AR, Aronstam EM. Unresectable squamous cell carcinoma of the lung and its management by combined bleomycin and radiotherapy. Cancer 1976;37(6):2671‐6. - PubMed
DasGupta 2006 {published data only}
-
- Dasgupta A, Dasgupta C, Basu S, Majumdar A. A prospective and randomized study of radiotherapy, sequential chemotherapy radiotherapy and concomitant chemotherapy‐radiotherapy in unresectable non small cell carcinoma of the lung. Journal of cancer research and therapeutics 2006;2(2):47‐51. - PubMed
Furuse 1999 {published data only}
-
- Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential radiotherapy in combination with mitomycin, vindesine and cisplatin in unresectable stage III non‐small cell lung cancer. Journal of Clinical Oncology 1999;17(9):2692‐9. - PubMed
Guschall 2000 {published data only}
-
- Gurschall W‐R, Schneemann‐Heinze S, Floegel R, Liebetrau G, Dittrich I. The therapy of the inoperable non small cell lung cancer: radiotherapy vs radio‐chemotherapy. Lung Cancer 2000;29 Suppl(1):115‐6.
Isakovic‐Vidovic2002 {published data only}
-
- Isakovic‐Vidovic S, Radosevic‐Jelic L, Borojevic N. Combined chemoradiotherapy in the treatment of locally advanced non‐small cell lung cancer. Journal of B.U.ON: official journal of the Balkan Union of Oncology 2002;7(1):47‐51. - PubMed
Japan ACNU 1989 {published data only}
-
- Japan Radiation‐ACNU Study Group. A randomized prospective study of radiation versus radiation plus ACNU in inoperable non‐small cell carcinoma of the lung. Cancer 1989;63(2):249‐54. - PubMed
Johnson 1990 {published data only}
-
- Johnson DH, Einhorn LH, Bartolucci A, Birch R, Omura G, Perez CA, et al. Thoracic radiotherapy does not prolong survival in patients with locally advanced, unresectable non‐small cell lung cancer. Annals of Internal Medicine 1990;113(1):33‐8. - PubMed
Koca 1996 {published data only}
-
- Koca S, Oner‐Dincbas F, Serdengecti S, Yaman M, Ongen G, Dimirci S, et al. Inoperabl kucuk hucreli disi akciger kanserinde hiperfraksiyone radyoterapi ile cisplatin+5FU kombinasyonunun yeri: randomize prospektif klinik calisma [Radiotherapy alone vs combined chemotherapy and radiotherapy in nonresectable non‐small cell lung cancer: a phase III randomized clinical trial]. Cerrahpasa J Med 1996;27:63‐69.
Komaki 2002 {published data only}
-
- Komaki R, Seiferheld W, Ettinger D, Lee JS, Mosvas B, Sause W. Randomized phase II chemotherapy and radiotherapy trial for patients with locally advanced inoperable non‐small‐cell lung cancer: long‐term follow‐up of RTOG 92‐04. International Journal of Radiation Oncology, Biology, Physics 2002;53(3):548‐57. - PubMed
LePar 1967 {published data only}
-
- LePar E, Faust DS, Brady LW, Beckloff GL. Clinical evaluation of the adjunctive use of hydroxyurea (NSC‐32065) in radiation therapy of carcinoma of the lung. Radiologia Clinica et Biologica 1967;36:32‐40. - PubMed
Misirlioglu 2006 {published data only}
-
- Misirlioglu CH, Erkal H, Elgin Y, Ugur I, Altundag K. Effect of concomitant use of pentoxifylline and alpha‐tocopherol with radiotherapy on the clinical outcome of patients with stage IIIB non‐small cell lung cancer: A randomized prospective clinical trial. Medical Oncology 2006;23(2):185‐9. - PubMed
Sarihan 2002 {published data only}
-
- Sarihan S, Kayisogullari U, Sozer N, Ercan I, Ozkan L, Engin K. A phase III study: results of radiotherapy alone versus radiotherapy with paclitaxel concomitantly in non‐small cell lung cancer. Radiotherapy and Oncology 2002;64 Suppl(1):258.
Ulutin 2000 {published data only}
-
- Ulutin HC, Guden M, Oysul K, Surenkok S, Pak Y. Split‐course radiotherapy with or without concurrent or sequential chemotherapy in non‐small cell lung cancer. Radiation Medicine 2000;18:93‐6. - PubMed
References to studies awaiting assessment
Zhang 2006 {published data only}
-
- Zhang Y, Gao X. [The clinical research on 10‐hydroxycamplothecin (HCPT) for radiosensitization on local advanced non‐small cell lung cancer]. Chinese Journal of Clinical Oncology 2006;33(8):458‐61.
References to ongoing studies
NCRI SOCCAR {published data only}
-
- SOCCAR Trial Cisplatin and Vinorelbine for NSCLC (LUWOS‐012/1). Ongoing study 01/12/2005.
Additional references
Auperin 2003
-
- Auperin A, Péchoux C. Meta‐analysis of randomized trials evaluating cisplatin or carboplatin‐based concomitant chemoradiation versus radiotherapy alone in locally advanced non‐small cell lung cancer (NSCLC). Lung Cancer 2003;41 Suppl(2):70.
Auperin 2007
-
- Auperin A, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, et al. Concomitant radio‐chemotherapy (RT‐CT) versus sequential RT‐CT in locally advanced non‐small cell lung cancer (NSCLC): a meta‐analysis using individual patient data (IPD) from randomised clinical trials (RCTs). Journal of Thoracic Oncology 2007;2(8):suppl 4 S310.
Auperin 2010
-
- Auperin A, Péchoux C, Rolland E, Curran W, Furuse K, Fournel P, et al. Meta‐Analysis of Concomitant Versus Sequential Radiochemotherapy in Locally Advanced Non–Small‐Cell Lung Cancer. Journal of Clinical Oncology 2010 Mar 29 [Epub ahead of print]. - PubMed
Ball 1999
-
- Ball D, Bishop J, Smith J, O'Brien P, Davis S, Ryan G, et al. A randomised phase II study of accelerated or standard fraction radiotherapy with or without concurrent carboplatin in inoperable non‐small cell lung cancer: final report of an Australian multi‐centre trial. Radiotherapy and Oncology 1999;52(2):129‐36. - PubMed
Cox 1990
-
- Cox JD, Azarnia N, Byhardt RW, Shin KH, Emami B, Pajak TF. A randomized phase I/II trial of hyperfractionated radiation therapy with total doses of 60.0Gy to 79.2Gy: possible survival benefit with greater than or equal to 69.6Gy in favorable patients with Radiation Therapy Oncology Group stage III non‐small cell lung carcinoma: report of Radiation Therapy Oncology Group 83‐11. Journal of Clinical Oncology 1990;8:1543‐55. - PubMed
Current Controlled Trials
-
- Biomed Central. Current Controlled Trials. www.controlled‐trials.com (accessed 19 October 2009).
DerSimonian 1986
-
- DerSimonian R. Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
El Sharouni 2003
Higgins 2009
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. [updated September 2009]. The Cochrane Collaboration, 2009.
Higgins 2009b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration, 2009.
Jones 2001
-
- Jones B, Dale RG, Deehan C, Hopkins KI, Morgan DA. The role of biologically effective dose (BED) in clinical oncology. Clinical Oncology 2001;13(2):71‐81. - PubMed
NSCLCCG 1995
NSCLCCG 2000
Parmar 1998
-
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. - PubMed
Perez 1987
-
- Perez CA, Pajak TF, Rubin P, Simpson JR, Mohiuddin M, Brady LW, et al. Long‐term observations of the patterns of failure in patients with unresectable non‐oat cell carcinoma of the lung treated with definitive radiotherapy. Cancer 1987;59:1874‐81. - PubMed
Rolland 2007
-
- Rolland E, Chevalier T, Auperin A, Burdett S, Pignon JP on behalf of the NSCLC Collaborative Group. Sequential radio‐chemotherapy(RT‐CT) versus radiotherapy alone (RT) and concomitant RT‐CT versus RTalone in locally advanced non‐small cell lung cancer (NSCLC): two metaanalyses using individual patient data (IPD) from randomised clinical trials(RCTs). Journal of Thoracic Oncology 2007;2(8):Suppl 4 S309‐10.
Saunders 1999
-
- Saunders M, Dische S, Barrett A, Harvey A, Griffiths G, Parmar M (on behalf of the CHART steering committee). Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non‐small cell lung cancer: mature data from the randomised multicentre trial. Radiotherapy and Oncology 1999;52:137‐48. - PubMed
References to other published versions of this review
Rowell 2004
-
- Rowell NP, O'Rourke NArt. No.: CD002140. DOI: 10.1002/14651858.CD002140.pub2. Concurrent chemoradiotherapy in non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2004, Issue 4. [Art. No.: CD002140. DOI: 10.1002/14651858.CD002140.pub2] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

