Origins of stereoselectivity in the trans Diels-Alder paradigm
- PMID: 20557046
- PMCID: PMC2908589
- DOI: 10.1021/ja1009162
Origins of stereoselectivity in the trans Diels-Alder paradigm
Abstract
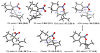
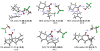
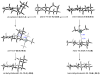
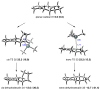
The regioselectivity and stereoselectivity aspects of the Diels-Alder/radical hydrodenitration reaction sequence leading to trans-fused ring systems have been investigated with density functional calculations. A continuum of transition structures representing Diels-Alder and hetero-Diels-Alder cycloadditions as well as a sigmatropic rearrangement have been located, and they all lie very close in energy on the potential energy surface. All three pathways are found to be important in the formation of the Diels-Alder adduct. Reported regioselectivities are reproduced by the calculations. The stereoselectivity of radical hydrodenitration of the cis-Diels-Alder adduct is found to be related to the relative conformational stabilities of bicyclic radical intermediates. Overall, the computations provide understanding of the regioselectivities and stereoselectivities of the trans-Diels-Alder paradigm.
Figures
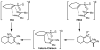




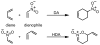

Similar articles
-
Conformational effects in diastereoselective aryne Diels-Alder reactions: synthesis of benzo-fused [2.2.1] heterobicycles.Org Lett. 2009 Oct 15;11(20):4688-91. doi: 10.1021/ol9019869. Org Lett. 2009. PMID: 19778014
-
Effect of Lewis acid catalysts on Diels-Alder and hetero-Diels-Alder cycloadditions sharing a common transition state.J Org Chem. 2008 Oct 3;73(19):7472-80. doi: 10.1021/jo801076t. Epub 2008 Sep 10. J Org Chem. 2008. PMID: 18781801
-
Origins of the Endo and Exo Selectivities in Cyclopropenone, Iminocyclopropene, and Triafulvene Diels-Alder Cycloadditions.J Org Chem. 2018 Mar 16;83(6):3164-3170. doi: 10.1021/acs.joc.8b00025. Epub 2018 Mar 7. J Org Chem. 2018. PMID: 29470085 Free PMC article.
-
The asymmetric hetero-Diels-Alder reaction in the syntheses of biologically relevant compounds.Angew Chem Int Ed Engl. 2014 Oct 13;53(42):11146-57. doi: 10.1002/anie.201404094. Epub 2014 Sep 12. Angew Chem Int Ed Engl. 2014. PMID: 25220929 Review.
-
Recent Advances in Inverse-Electron-Demand Hetero-Diels-Alder Reactions of 1-Oxa-1,3-Butadienes.Top Curr Chem (Cham). 2016 Jun;374(3):24. doi: 10.1007/s41061-016-0026-2. Epub 2016 Apr 20. Top Curr Chem (Cham). 2016. PMID: 27573264 Free PMC article. Review.
Cited by
-
Mechanistic elucidation of the tandem Diels-Alder/(3 + 2) cycloadditions in the design and syntheses of heterosteroids.J Mol Model. 2022 Feb 27;28(3):70. doi: 10.1007/s00894-022-05063-5. J Mol Model. 2022. PMID: 35220485
-
Orthogonal Inverse-Electron-Demand Cycloaddition Reactions Controlled by Frontier Molecular Orbital Interactions.Org Lett. 2023 Sep 1;25(34):6340-6345. doi: 10.1021/acs.orglett.3c02265. Epub 2023 Aug 17. Org Lett. 2023. PMID: 37591496 Free PMC article.
-
Ring-opening carbonyl-olefin metathesis of norbornenes.Chem Sci. 2020 Jul 1;11(30):7884-7895. doi: 10.1039/d0sc02243h. Chem Sci. 2020. PMID: 34094159 Free PMC article.
-
Investigating the site-, regio-, and stereo-selectivities of the reactions between organic azide and 7-heteronorbornadiene: a DFT mechanistic study.J Mol Model. 2021 Aug 13;27(9):248. doi: 10.1007/s00894-021-04857-3. J Mol Model. 2021. PMID: 34387742
-
Synthesis of Angularly Substituted trans-Fused Decalins through a Metallacycle-Mediated Annulative Cross-Coupling Cascade.Angew Chem Int Ed Engl. 2016 Oct 10;55(42):13099-13103. doi: 10.1002/anie.201606962. Angew Chem Int Ed Engl. 2016. PMID: 27634059 Free PMC article.
References
-
- Denmark SE, Thorarensen A. Chem Rev. 1996;96:137–165. - PubMed
-
- Denmark SE, Kesler BS, Moon YC. J Org Chem. 1992;57:4912–4924.
-
- Denmark SE, Cramer CJ, Sternberg JA. Helv Chim Acta. 1986;69:1971–1989.
- Denmark SE, Cramer CJ, Sternberg JA. Tetrahedron Lett. 1986;27:3693–3696.
-
- Denmark SE, Moon YC, Senanayake CBW. J Am Chem Soc. 1990;112:311–315.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

