Monocyte chemoattractant protein-1 is generated via TGF-beta by myofibroblasts in gastric intestinal metaplasia and carcinoma without H. pylori infection
- PMID: 20557309
- PMCID: PMC11158495
- DOI: 10.1111/j.1349-7006.2010.01609.x
Monocyte chemoattractant protein-1 is generated via TGF-beta by myofibroblasts in gastric intestinal metaplasia and carcinoma without H. pylori infection
Abstract
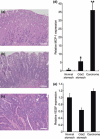
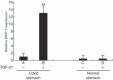
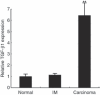
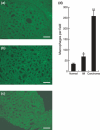
Helicobacter pylori (H. pylori) stimulates secretion of monocyte chemoattractant protein 1 (MCP-1) from gastric mucosa. Monocyte chemoattractant protein-1 (MCP-1) expression and macrophage infiltration are recognized in human gastric carcinoma. We have previously generated Cdx2-transgenic mice as model mice for intestinal metaplasia. Both chronic H. pylori-associated gastritis and Cdx2-transgenic mouse stomach develop intestinal metaplasia and finally gastric carcinoma. In this study we have directed our attention to MCP-1 expression in the intestinal metaplastic mucosa and the gastric carcinoma of Cdx2-transgenic mouse stomach. Quantitative real-time PCR was performed to determine MCP-1 and transforming growth factor-beta1 (TGF-beta1) mRNA expression levels and single- or double-label immunohistochemistry was used to evaluate the localization of MCP-1, TGF-beta type I receptor, and alpha-smooth muscle actin (alphaSMA). We determined that MCP-1 mRNA dramatically increased in the intestinal metaplastic mucosa and the gastric carcinoma of Cdx2-transgenic mouse stomach, compared with normal mouse stomach. Both MCP-1 and TGF-beta type I receptor were co-expressed in the alphaSMA-positive myofibroblasts of intestinal metaplastic mucosa and gastric carcinoma. Exogenous application of TGF-beta1 increased MCP-1 mRNA expression levels in the intestinal metaplastic tissue. Furthermore, TGF-beta1 was overexpressed and macrophage was strongly infiltrated in the gastric carcinoma. In conclusion, MCP-1 expression, which was stimulated by TGF-beta1, was recognized in the TGF-beta type I receptor-expressing myofibroblasts of the intestinal metaplastic mucosa and the gastric carcinoma of Cdx2-transgenic mouse stomach. The present results suggest that intestinal metaplasia and gastric carcinoma themselves induce MCP-1 expression independently of H. pylori infection.
Figures


Similar articles
-
Cdx1 induced intestinal metaplasia in the transgenic mouse stomach: comparative study with Cdx2 transgenic mice.Gut. 2004 Oct;53(10):1416-23. doi: 10.1136/gut.2003.032482. Gut. 2004. PMID: 15361487 Free PMC article.
-
Sox2 expression is maintained while gastric phenotype is completely lost in Cdx2-induced intestinal metaplastic mucosa.Differentiation. 2011 Feb;81(2):92-8. doi: 10.1016/j.diff.2010.10.002. Epub 2010 Oct 30. Differentiation. 2011. PMID: 21036460
-
Transgenic Cdx2 induces endogenous Cdx1 in intestinal metaplasia of Cdx2-transgenic mouse stomach.FEBS J. 2009 Oct;276(20):5821-31. doi: 10.1111/j.1742-4658.2009.07263.x. Epub 2009 Sep 2. FEBS J. 2009. PMID: 19725873
-
Atrophy-metaplasia-dysplasia-carcinoma sequence in the stomach: a reality or merely an hypothesis?Best Pract Res Clin Gastroenterol. 2001 Dec;15(6):983-98. doi: 10.1053/bega.2001.0253. Best Pract Res Clin Gastroenterol. 2001. PMID: 11866488 Review.
-
[Intestinal metaplasia of the gastric mucosa and its relation with gastric carcinoma].G E N. 1994 Jul-Sep;48(3):190-4. G E N. 1994. PMID: 7768424 Review. Spanish.
Cited by
-
Sex-specific effects of Eugenia punicifolia extract on gastric ulcer healing in rats.World J Gastroenterol. 2018 Oct 14;24(38):4369-4383. doi: 10.3748/wjg.v24.i38.4369. World J Gastroenterol. 2018. PMID: 30344421 Free PMC article.
-
Elevated circulating TGFβ1 during acute liver failure activates TGFβR2 on cortical neurons and exacerbates neuroinflammation and hepatic encephalopathy in mice.J Neuroinflammation. 2019 Apr 2;16(1):69. doi: 10.1186/s12974-019-1455-y. J Neuroinflammation. 2019. PMID: 30940161 Free PMC article.
-
Increased numbers of Foxp3-positive regulatory T cells in gastritis, peptic ulcer and gastric adenocarcinoma.World J Gastroenterol. 2012 Jan 7;18(1):34-43. doi: 10.3748/wjg.v18.i1.34. World J Gastroenterol. 2012. PMID: 22228968 Free PMC article.
-
Gastric Sonic Hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori.Gastroenterology. 2012 May;142(5):1150-1159.e6. doi: 10.1053/j.gastro.2012.01.029. Epub 2012 Jan 25. Gastroenterology. 2012. PMID: 22285806 Free PMC article.
-
Increased expression of C-C motif ligand 2 associates with poor prognosis in patients with gastric cancer after gastrectomy.Tumour Biol. 2016 Mar;37(3):3285-93. doi: 10.1007/s13277-015-4092-9. Epub 2015 Oct 5. Tumour Biol. 2016. PMID: 26438062
References
-
- Baggiolini M, Loetscher P, Moser B. Interleukin‐8 and the chemokine family. Int J Immunopharmacol 1995; 17: 103–8. - PubMed
-
- Watanabe N, Shimada T, Ohtsuka Y, Hiraishi H, Terano A. Proinflammatory cytokines and Helicobacter pylori stimulate CC‐chemokine expression in gastric epithelial cells. J Physiol Pharmacol 1997; 48: 405–13. - PubMed
-
- Noach LA, Bosma NB, Jansen J, Hoek FJ, Van Deventer SJ, Tytgat GN. Mucosal tumor necrosis factor‐alpha, interleukin‐1 beta, and interleukin‐8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol 1994; 29: 425–9. - PubMed
-
- Jung HC, Kim JM, Song IS, Kim CY. Helicobacter pylori induces an array of pro‐inflammatory cytokines in human gastric epithelial cells: quantification of mRNA for interleukin‐8, ‐1 alpha/beta, granulocyte‐macrophage colony‐stimulating factor, monocyte chemoattractant protein‐1 and tumour necrosis factor‐alpha. J Gastroenterol Hepatol 1997; 12: 473–80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

