Activation of brain endothelium by pneumococcal neuraminidase NanA promotes bacterial internalization
- PMID: 20557315
- PMCID: PMC2943548
- DOI: 10.1111/j.1462-5822.2010.01490.x
Activation of brain endothelium by pneumococcal neuraminidase NanA promotes bacterial internalization
Abstract
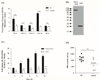
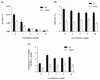
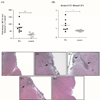
Streptococcus pneumoniae (SPN), the leading cause of meningitis in children and adults worldwide, is associated with an overwhelming host inflammatory response and subsequent brain injury. Here we examine the global response of the blood-brain barrier to SPN infection and the role of neuraminidase A (NanA), an SPN surface anchored protein recently described to promote central nervous system tropism. Microarray analysis of human brain microvascular endothelial cells (hBMEC) during infection with SPN or an isogenic NanA-deficient (ΔnanA) mutant revealed differentially activated genes, including neutrophil chemoattractants IL-8, CXCL-1, CXCL-2. Studies using bacterial mutants, purified recombinant NanA proteins and in vivo neutrophil chemotaxis assays indicated that pneumococcal NanA is necessary and sufficient to activate host chemokine expression and neutrophil recruitment during infection. Chemokine induction was mapped to the NanA N-terminal lectin-binding domain with a limited contribution of the sialidase catalytic activity, and was not dependent on the invasive capability of the organism. Furthermore, pretreatment of hBMEC with recombinant NanA protein significantly increased bacterial invasion, suggesting that NanA-mediated activation of hBMEC is a prerequisite for efficient SPN invasion. These findings were corroborated in an acute murine infection model where we observed less inflammatory infiltrate and decreased chemokine expression following infection with the ΔnanA mutant.
© 2010 Blackwell Publishing Ltd.
Figures


References
-
- Bernatoniene J, Zhang Q, Dogan S, Mitchell TJ, Paton JC, Finn A. Induction of CC and CXC chemokines in human antigen-presenting dendritic cells by the pneumococcal proteins pneumolysin and CbpA, and the role played by toll-like receptor 4, NF-kappaB, and mitogen-activated protein kinases. J Infect Dis. 2008;198:1823–1833. - PubMed
-
- Betz AL. An overview of the multiple functions of the blood-brain barrier. NIDA Res Monogr. 1992;120:54–72. - PubMed
-
- Betz AL, Goldstein GW. Specialized properties and solute transport in brain capillaries. Annu Rev Physiol. 1986;48:241–250. - PubMed
-
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78:206–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

