Altered neurotransmission in the mesolimbic reward system of Girk mice
- PMID: 20557431
- PMCID: PMC2941778
- DOI: 10.1111/j.1471-4159.2010.06864.x
Altered neurotransmission in the mesolimbic reward system of Girk mice
Abstract
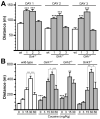
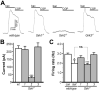
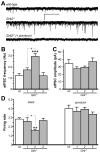
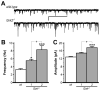
Mice lacking the Girk2 subunit of G protein-gated inwardly rectifying K+ (Girk) channels exhibit dopamine-dependent hyperactivity and elevated responses to drugs that stimulate dopamine neurotransmission. The dopamine-dependent phenotypes seen in Girk2(-/-) mice could reflect increased intrinsic excitability of or diminished inhibitory feedback to midbrain dopamine neurons, or secondary adaptations triggered by Girk2 ablation. We addressed these possibilities by evaluating Girk(-/-) mice in behavioral, electrophysiological, and cell biological assays centered on the mesolimbic dopamine system. Despite differences in the contribution of Girk1 and Girk2 subunits to Girk signaling in midbrain dopamine neurons, Girk1(-/-) and Girk2(-/-) mice exhibited comparable baseline hyperactivities and enhanced responses to cocaine. Girk ablation also correlated with altered afferent input to dopamine neurons in the ventral tegmental area. Dopamine neurons from Girk1(-/-) and Girk2(-/-) mice exhibited elevated glutamatergic neurotransmission, paralleled by increased synaptic levels of alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate glutamate receptors. In addition, synapse density, alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptor levels, and glutamatergic neurotransmission were elevated in medium spiny neurons of the nucleus accumbens from Girk1(-/-) and Girk2(-/-) mice. We conclude that dopamine-dependent phenotypes in Girk2(-/-) mice are not solely attributable to a loss of Girk signaling in dopamine neurons, and likely involve secondary adaptations facilitating glutamatergic signaling in the mesolimbic reward system.
Figures



References
-
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. - PubMed
-
- Bettahi I, Marker CL, Roman MI, Wickman K. Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel IKACh. J Biol Chem. 2002;277:48282–48288. - PubMed
-
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. - PubMed
-
- Blednov YA, Stoffel M, Chang SR, Harris RA. GIRK2 deficient mice. Evidence for hyperactivity and reduced anxiety. Physiol Behav. 2001;74:109–117. - PubMed
-
- Blednov YA, Stoffel M, Cooper R, Wallace D, Mane N, Harris RA. Hyperactivity and dopamine D1 receptor activation in mice lacking girk2 channels. Psychopharmacology (Berl) 2002;159:370–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

