Cloning and distribution of a putative octopamine/tyramine receptor in the central nervous system of the freshwater prawn Macrobrachium rosenbergii
- PMID: 20558147
- PMCID: PMC2920218
- DOI: 10.1016/j.brainres.2010.06.021
Cloning and distribution of a putative octopamine/tyramine receptor in the central nervous system of the freshwater prawn Macrobrachium rosenbergii
Abstract
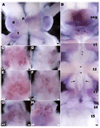
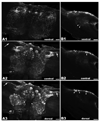
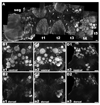
There is ample evidence linking octopamine (OA) and tyramine (TA) to several neurophysiological functions in arthropods. In our laboratory we use the freshwater prawn Macrobrachium rosenbergii to study the neural basis of aggressive behavior. As a first step towards understanding the possible role of these amines and their receptors in the modulation of interactive behaviors, we have cloned a putative octopamine/tyramine receptor. The predicted sequence of the cloned OA/TA(Mac) receptor consists of 1,579 base pairs (bp), with an open reading frame of 1,350bp that encodes a 450 amino acid protein. This putative protein displays sequence identities of 70% to an Aedes aegypti mosquito TA receptor, followed by 60% to a Stegomyia aegypti mosquito OA receptor, 59% and 58% to the migratory locust TA-1 and -2 receptors respectively, and 57% with the silkworm OA receptor. We also mapped the OA/TA(Mac) receptor distribution by in-situ hybridization to the receptor's mRNA, and by immunohistochemistry to its protein. We observed stained cell bodies for the receptor's mRNA, mainly in the midline region of the thoracic and in the abdominal ganglia, as well as diffuse staining in the brain ganglia. For the receptor's protein, we observed extensive punctate staining within the neuropil and on the membrane of specific groups of neurons in all ganglia throughout the CNS, including the brain, the midline region and neuropiles of the thoracic ganglia, and ventral part and neuropiles of the abdominal ganglia. The same pattern of stained cells was observed on the thoracic and abdominal ganglia in both in-situ hybridization and immunohistochemistry experiments. Diffuse staining observed with in-situ hybridization also coincides with punctate staining observed in brain, SEG, thoracic, and abdominal ganglia in immunohistochemical preparations. This work provides the first step towards characterizing the neural networks that mediate octopaminergic signaling in prawn.
Published by Elsevier B.V.
Figures

References
-
- Antonsen BL, Paul DH. Serotonergic and octopaminergic systems in the squat lobster Munida quadrispina (Anomura, Glatheidae) J Comp Neurol. 2001;439:450–468. - PubMed
-
- Arakawa S, Gocayne JD, McCombie WR, Urquhart DA, Hall LM, Fraser CM, Vanter JC. Cloning, localization, and permanent expression of a Drosophila octopamine receptor. Neuron. 1990;4:343–354. - PubMed
-
- Axelrod J, Saavedra JM. Octopamine. Nature. 1977;265:501–504. - PubMed
-
- Barki A, Karplus I, Goren M. The agonistic behavior of the three male morphotypes of the freshwater prawn Macrobrachium rosenbergii (Crustacea, Palaemonidae) Behavior. 1991a;116:252–277.
-
- Barki A, Karplus I, Goren M. Morphotype related dominance hierarchies in males of Macrobrachium rosenbergii (Crustacea, Palaemoidae) Behavior. 1991b;117:145–160.
Publication types
MeSH terms
Substances
Grants and funding
- NIH RO1 NS39103/NS/NINDS NIH HHS/United States
- R24 MH048190/MH/NIMH NIH HHS/United States
- R25 GM061838/GM/NIGMS NIH HHS/United States
- R01 NS064259/NS/NINDS NIH HHS/United States
- S06GM008224/GM/NIGMS NIH HHS/United States
- G12RR0305/RR/NCRR NIH HHS/United States
- G12 RR003051/RR/NCRR NIH HHS/United States
- S06 GM008224/GM/NIGMS NIH HHS/United States
- P20 GM103642/GM/NIGMS NIH HHS/United States
- G12 MD007600/MD/NIMHD NIH HHS/United States
- R25-GM061838/GM/NIGMS NIH HHS/United States
- R01 NS039103/NS/NINDS NIH HHS/United States
- SC3 GM084763/GM/NIGMS NIH HHS/United States
- G12 RR003050/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

