New monoclonal anti-mouse DC-SIGN antibodies reactive with acetone-fixed cells
- PMID: 20558171
- PMCID: PMC2924951
- DOI: 10.1016/j.jim.2010.06.006
New monoclonal anti-mouse DC-SIGN antibodies reactive with acetone-fixed cells
Abstract
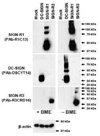
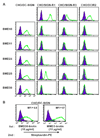
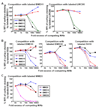
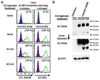
Mouse DC-SIGN CD209a is a type II transmembrane protein, one of a family of C-type lectin genes syntenic and homologous to human DC-SIGN. Current anti-mouse DC-SIGN monoclonal antibodies (MAbs) are unable to react with DC-SIGN in acetone-fixed cells, limiting the chance to visualize DC-SIGN in tissue sections. We first produced rabbit polyclonal PAb-DSCYT14 against a 14-aa peptide in the cytosolic domain of mouse DC-SIGN, and it specifically detected DC-SIGN and not the related lectins, SIGN-R1 and SIGN-R3 expressed in transfected CHO cells. MAbs were generated by immunizing rats and DC-SIGN knockout mice with the extracellular region of mouse DC-SIGN. Five rat IgG2a or IgM MAbs, named BMD10, 11, 24, 25, and 30, were selected and each MAb specifically detected DC-SIGN by FACS and Western blots, although BMD25 was cross-reactive to SIGN-R1. Two mouse IgG2c MAbs MMD2 and MMD3 interestingly bound mouse DC-SIGN but at 10 fold higher levels than the rat MAbs. When the binding epitopes of the new BMD and two other commercial rat anti-DC-SIGN MAbs, 5H10 and LWC06, were examined by competition assays, the epitopes of BMD11, 24, and LWC06 were identical or closely overlapping while BMD10, 30, and 5H10 were shown to bind different epitopes. MMD2 and MMD3 epitopes were on a 3rd noncompeting region of mouse DC-SIGN. DC-SIGN expressed on the cell surface was sensitive to collagenase treatment, as monitored by polyclonal and MAb. These new reagents should be helpful to probe the biology of DC-SIGN in vivo.
2010 Elsevier B.V. All rights reserved.
Figures
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Caminschi I, Corbett AJ, Zahra C, Lahoud M, Lucas KM, Sofi M, Vremec D, Gramberg T, Pohlmann S, Curtis J, Handman E, van Dommelen SL, Fleming P, Degli-Esposti MA, Shortman K, Wright MD. Functional comparison of mouse CIRE/mouse DC-SIGN and human DC-SIGN. Int. Immunol. 2006;18:741–753. - PMC - PubMed
-
- Caminschi I, Lucas KM, O'Keeffe MA, Hochrein H, Laabi Y, Brodnicki TC, Lew AM, Shortman K, Wright MD. Molecular cloning of a C-type lectin superfamily protein differentially expressed by CD8α− splenic dendritic cells. Mol. Immunol. 2001;38:365–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

