Down's syndrome-like cardiac developmental defects in embryos of the transchromosomic Tc1 mouse
- PMID: 20558441
- PMCID: PMC2952533
- DOI: 10.1093/cvr/cvq193
Down's syndrome-like cardiac developmental defects in embryos of the transchromosomic Tc1 mouse
Abstract
Aims: Cardiac malformations are prevalent in trisomies of human chromosome 21 [Down's syndrome (DS)], affecting normal chamber separation in the developing heart. Efforts to understand the aetiology of these defects have been severely hampered by the absence of an accurate mouse model. Such models have proved challenging to establish because synteny with human chromosome Hsa21 is distributed across three mouse chromosomes. None of those engineered so far accurately models the full range of DS cardiac phenotypes, in particular the profound disruptions resulting from atrioventricular septal defects (AVSDs). Here, we present analysis of the cardiac malformations exhibited by embryos of the transchromosomic mouse line Tc(Hsa21)1TybEmcf (Tc1) which contains more than 90% of chromosome Hsa21 in addition to the normal diploid mouse genome.
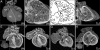
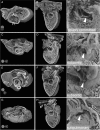
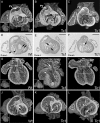
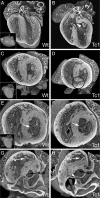
Methods and results: Using high-resolution episcopic microscopy and three-dimensional (3D) modelling, we show that Tc1 embryos exhibit many of the cardiac defects found in DS, including balanced AVSD with single and separate valvar orifices, membranous and muscular ventricular septal defects along with outflow tract and valve leaflet abnormalities. Frequencies of cardiac malformations (ranging from 38 to 55%) are dependent on strain background. In contrast, no comparable cardiac defects were detected in embryos of the more limited mouse trisomy model, Dp(16Cbr1-ORF9)1Rhr (Ts1Rhr), indicating that trisomy of the region syntenic to the Down's syndrome critical region, including the candidate genes DSCAM and DYRK1A, is insufficient to yield DS cardiac abnormalities.
Conclusion: The Tc1 mouse line provides a suitable model for studying the underlying genetic causes of the DS AVSD cardiac phenotype.
Figures

Comment in
-
Looking down the atrioventricular canal.Cardiovasc Res. 2010 Nov 1;88(2):205-6. doi: 10.1093/cvr/cvq302. Epub 2010 Sep 20. Cardiovasc Res. 2010. PMID: 20855523 Free PMC article. No abstract available.
References
-
- Sherman SL, Allen EG, Bean LH, Freeman SB. Epidemiology of Down syndrome. Ment Retard Dev Disabil Res Rev. 2007;13:221–227. doi:10.1002/mrdd.20157. - DOI - PubMed
-
- Freeman SB, Taft LF, Dooley KJ, Allran K, Sherman SL, Hassold TJ, et al. Population-based study of congenital heart defects in Down syndrome. Am J Med Genet. 1998;80:213–217. doi:10.1002/(SICI)1096-8628(19981116)80:3<213::AID-AJMG6>3.0.CO;2-8. - DOI - PubMed
-
- Korenberg JR, Chen XN, Schipper R, Sun Z, Gonsky R, Gerwehr S, et al. Down syndrome phenotypes: the consequences of chromosomal imbalance. Proc Natl Acad Sci USA. 1994;91:4997–5001. doi:10.1073/pnas.91.11.4997. - DOI - PMC - PubMed
-
- Delabar JM, Theophile D, Rahmani Z, Chettouh Z, Blouin JL, Prieur M, et al. Molecular mapping of twenty-four features of Down syndrome on chromosome 21. Eur J Hum Genet. 1993;1:114–124. - PubMed
-
- Korbel JO, Tirosh-Wagner T, Urban AE, Chen XN, Kasowski M, Dai L, et al. The genetic architecture of Down syndrome phenotypes revealed by high-resolution analysis of human segmental trisomies. Proc Natl Acad Sci USA. 2009;106:12031–12036. doi:10.1073/pnas.0813248106. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

