Consecutive pharmacological activation of PKA and PKC mimics the potent cardioprotection of temperature preconditioning
- PMID: 20558443
- PMCID: PMC2952531
- DOI: 10.1093/cvr/cvq190
Consecutive pharmacological activation of PKA and PKC mimics the potent cardioprotection of temperature preconditioning
Abstract
Aims: Temperature preconditioning (TP) provides very powerful protection against ischaemia/reperfusion. Understanding the signalling pathways involved may enable the development of effective pharmacological cardioprotection. We investigated the interrelationship between activation of protein kinase A (PKA) and protein kinase C (PKC) in the signalling mechanisms of TP and developed a potent pharmacological intervention based on this mechanism.
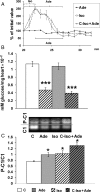
Methods and results: Isolated rat hearts were subjected to TP, 30 min global ischaemia, and 60 min reperfusion. Other control and TP hearts were perfused with either sotalol (β-adrenergic blocker) or H-89 (PKA inhibitor). Some hearts were pre-treated with either isoproterenol (β-adrenergic agonist) or adenosine (PKC activator) that were given alone, simultaneously, or sequentially. Pre-treatment with isoproterenol, adenosine, and the consecutive isoproterenol/adenosine treatment was also combined with the PKC inhibitor chelerythrine. Cardioprotection was evaluated by haemodynamic function recovery, lactate dehydrogenase release, measurement of mitochondrial permeability transition pore opening, and protein carbonylation during reperfusion. Cyclic AMP and PKA activity were increased in TP hearts. H-89 and sotalol blocked the cardioprotective effect of TP and TP-induced PKC activation. Isoproterenol, adenosine, and the consecutive treatment increased PKC activity during pre-ischaemia. Isoproterenol significantly reduced myocardial glycogen content. Isoproterenol and adenosine, alone or simultaneously, protected hearts but the consecutive treatment gave the highest protection. Cardioprotective effects of adenosine were completely blocked by chelerythrine but those of the consecutive treatment only attenuated.
Conclusion: The signal transduction pathway of TP involves PKA activation that precedes PKC activation. Pharmacologically induced consecutive PKA/PKC activation mimics TP and induces extremely potent cardioprotection.
Figures



References
-
- Depre C, Taegtmeyer H. Metabolic aspects of programmed cell survival and cell death in the heart. Cardiovasc Res. 2000;45:538–548. doi:10.1016/S0008-6363(99)00266-7. - DOI - PubMed
-
- Khaliulin I, Clarke SJ, Lin H, Parker JE, Suleiman MS, Halestrap AP. Temperature preconditioning of isolated rat hearts—a potent cardioprotective mechanism involving a reduction in oxidative stress and inhibition of the mitochondrial permeability transition pore. J Physiol. 2007;581:1147–1161. doi:10.1113/jphysiol.2007.130369. - DOI - PMC - PubMed
-
- Clarke SJ, Khaliulin I, Das M, Parker JE, Heesom KJ, Halestrap AP. Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ Res. 2008;102:1082–1090. doi:10.1161/CIRCRESAHA.107.167072. - DOI - PMC - PubMed
-
- Lochner A, Genade S, Tromp E, Podzuweit T, Moolman JA. Ischemic preconditioning and the β-adrenergic signal transduction pathway. Circulation. 1999;100:958–966. - PubMed
-
- Yellon DM, Dana A. The preconditioning phenomenon: a tool for the scientist or a clinical reality? Circ Res. 2000;87:543–550. - PubMed

