Mitochondrial calcium uptake capacity as a therapeutic target in the R6/2 mouse model of Huntington's disease
- PMID: 20558522
- PMCID: PMC2916705
- DOI: 10.1093/hmg/ddq247
Mitochondrial calcium uptake capacity as a therapeutic target in the R6/2 mouse model of Huntington's disease
Abstract
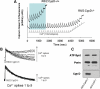
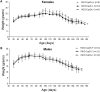
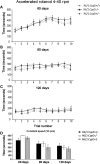
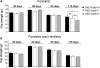
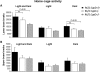
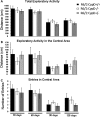
Huntington's disease (HD) is an incurable autosomal-dominant neurodegenerative disorder initiated by an abnormally expanded polyglutamine domain in the huntingtin protein. It is proposed that abnormal mitochondrial Ca2+ capacity results in an increased susceptibility to mitochondrial permeability transition (MPT) induction that may contribute significantly to HD pathogenesis. The in vivo contribution of these hypothesized defects remains to be elucidated. In this proof-of-principle study, we examined whether increasing mitochondrial Ca2+ capacity could ameliorate the well-characterized phenotype of the R6/2 transgenic mouse model. Mouse models lacking cyclophilin D demonstrate convincingly that cyclophilin D is an essential component and a key regulator of MPT induction. Mitochondria of cyclophilin D knockout mice are particularly resistant to Ca2+ overload. We generated R6/2 mice with normal, reduced or absent cyclophilin D expression and examined the effect of increasing mitochondrial Ca2+ capacity on the behavioral and neuropathological features of the R6/2 model. A predicted outcome of this approach was the finding that cyclophilin D deletion enhanced the R6/2 brain mitochondria Ca2+ capacity significantly. Increased neuronal mitochondrial Ca2+ capacity failed to ameliorate either the behavioral and neuropathological features of R6/2 mice. We found no alterations in body weight changes, lifespan, RotaRod performances, grip strength, overall activity and no significant effect on the neuropathological features of R6/2 mice. The results of this study demonstrate that increasing neuronal mitochondrial Ca2+-buffering capacity is not beneficial in the R6/2 mouse model of HD.
Figures



References
-
- Folstein S.E. Huntington's Disease: a Disorder of Families. Baltimore: Johns Hopkins University Press; 1989.
-
- Hunter J.M., Crouse A.B., Lesort M., Johnson G.V., Detloff P.J. Verification of somatic CAG repeat expansion by pre-PCR fractionation. J. Neurosci. Methods. 2005;144:11–17. doi:10.1016/j.jneumeth.2004.10.006. - DOI - PubMed
-
- Trottier Y., Biancalana V., Mandel J.L. Instability of CAG repeats in Huntington's disease: relation to parental transmission and age of onset. J. Med. Genet. 1994;31:377–382. doi:10.1136/jmg.31.5.377. - DOI - PMC - PubMed
-
- Telenius H., Kremer B., Goldberg Y.P., Theilmann J., Andrew S.E., Zeisler J., Adam S., Greenberg C., Ives E.J., Clarke L.A., et al. Somatic and gonadal mosaicism of the Huntington disease gene CAG repeat in brain and sperm. Nat. Genet. 1994;6:409–414. doi:10.1038/ng0494-409. - DOI - PubMed
-
- Kennedy L., Evans E., Chen C.M., Craven L., Detloff P.J., Ennis M., Shelbourne P.F. Dramatic tissue-specific mutation length increases are an early molecular event in Huntington disease pathogenesis. Hum. Mol. Genet. 2003;12:3359–3367. doi:10.1093/hmg/ddg352. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

