Target RNA-directed trimming and tailing of small silencing RNAs
- PMID: 20558712
- PMCID: PMC2902985
- DOI: 10.1126/science.1187058
Target RNA-directed trimming and tailing of small silencing RNAs
Abstract
In Drosophila, microRNAs (miRNAs) typically guide Argonaute1 to repress messenger RNA (mRNA), whereas small interfering RNAs (siRNAs) guide Argonaute2 to destroy viral and transposon RNA. Unlike siRNAs, miRNAs rarely form extensive numbers of base pairs to the mRNAs they regulate. We find that extensive complementarity between a target RNA and an Argonaute1-bound miRNA triggers miRNA tailing and 3'-to-5' trimming. In flies, Argonaute2-bound small RNAs--but not those bound to Argonaute1--bear a 2'-O-methyl group at their 3' ends. This modification blocks target-directed small RNA remodeling: In flies lacking Hen1, the enzyme that adds the 2'-O-methyl group, Argonaute2-associated siRNAs are tailed and trimmed. Target complementarity also affects small RNA stability in human cells. These results provide an explanation for the partial complementarity between animal miRNAs and their targets.
Figures
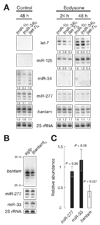
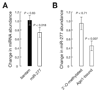



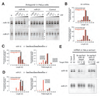
Comment in
-
Molecular biology. Paring miRNAs through pairing.Science. 2010 Jun 18;328(5985):1494-5. doi: 10.1126/science.1191531. Science. 2010. PMID: 20558697 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

