Selective translational control of the Alzheimer amyloid precursor protein transcript by iron regulatory protein-1
- PMID: 20558735
- PMCID: PMC2951196
- DOI: 10.1074/jbc.M110.149161
Selective translational control of the Alzheimer amyloid precursor protein transcript by iron regulatory protein-1
Abstract
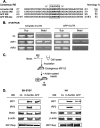
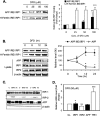
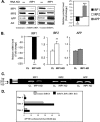
Iron influx increases the translation of the Alzheimer amyloid precursor protein (APP) via an iron-responsive element (IRE) RNA stem loop in its 5'-untranslated region. Equal modulated interaction of the iron regulatory proteins (IRP1 and IRP2) with canonical IREs controls iron-dependent translation of the ferritin subunits. However, our immunoprecipitation RT-PCR and RNA binding experiments demonstrated that IRP1, but not IRP2, selectively bound the APP IRE in human neural cells. This selective IRP1 interaction pattern was evident in human brain and blood tissue from normal and Alzheimer disease patients. We computer-predicted an optimal novel RNA stem loop structure for the human, rhesus monkey, and mouse APP IREs with reference to the canonical ferritin IREs but also the IREs encoded by erythroid heme biosynthetic aminolevulinate synthase and Hif-2α mRNAs, which preferentially bind IRP1. Selective 2'-hydroxyl acylation analyzed by primer extension analysis was consistent with a 13-base single-stranded terminal loop and a conserved GC-rich stem. Biotinylated RNA probes deleted of the conserved CAGA motif in the terminal loop did not bind to IRP1 relative to wild type probes and could no longer base pair to form a predicted AGA triloop. An AGU pseudo-triloop is key for IRP1 binding to the canonical ferritin IREs. RNA probes encoding the APP IRE stem loop exhibited the same high affinity binding to rhIRP1 as occurs for the H-ferritin IRE (35 pm). Intracellular iron chelation increased binding of IRP1 to the APP IRE, decreasing intracellular APP expression in SH-SY5Y cells. Functionally, shRNA knockdown of IRP1 caused increased expression of neural APP consistent with IRP1-APP IRE-driven translation.
Figures


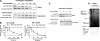

Similar articles
-
Iron response elements (IREs)-mRNA of Alzheimer's amyloid precursor protein binding to iron regulatory protein (IRP1): a combined molecular docking and spectroscopic approach.Sci Rep. 2023 Mar 28;13(1):5073. doi: 10.1038/s41598-023-32073-x. Sci Rep. 2023. PMID: 36977734 Free PMC article.
-
Novel 5' untranslated region directed blockers of iron-regulatory protein-1 dependent amyloid precursor protein translation: implications for down syndrome and Alzheimer's disease.PLoS One. 2013 Jul 31;8(7):e65978. doi: 10.1371/journal.pone.0065978. Print 2013. PLoS One. 2013. PMID: 23935819 Free PMC article.
-
Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer's disease.Biochem Soc Trans. 2008 Dec;36(Pt 6):1282-7. doi: 10.1042/BST0361282. Biochem Soc Trans. 2008. PMID: 19021541 Free PMC article. Review.
-
An iron-responsive element type II in the 5'-untranslated region of the Alzheimer's amyloid precursor protein transcript.J Biol Chem. 2002 Nov 22;277(47):45518-28. doi: 10.1074/jbc.M207435200. Epub 2002 Aug 26. J Biol Chem. 2002. PMID: 12198135
-
Iron-Mediated Overexpression of Amyloid Precursor Protein via Iron Responsive mRNA in Alzheimer's Disease.Int J Mol Sci. 2025 May 30;26(11):5283. doi: 10.3390/ijms26115283. Int J Mol Sci. 2025. PMID: 40508094 Free PMC article. Review.
Cited by
-
Brain iron metabolism and its perturbation in neurological diseases.J Neural Transm (Vienna). 2011 Mar;118(3):301-14. doi: 10.1007/s00702-010-0470-z. Epub 2010 Sep 1. J Neural Transm (Vienna). 2011. PMID: 20809066 Review.
-
Targeting the Iron-Response Elements of the mRNAs for the Alzheimer's Amyloid Precursor Protein and Ferritin to Treat Acute Lead and Manganese Neurotoxicity.Int J Mol Sci. 2019 Feb 25;20(4):994. doi: 10.3390/ijms20040994. Int J Mol Sci. 2019. PMID: 30823541 Free PMC article. Review.
-
Link between Aluminum and the Pathogenesis of Alzheimer's Disease: The Integration of the Aluminum and Amyloid Cascade Hypotheses.Int J Alzheimers Dis. 2011 Mar 8;2011:276393. doi: 10.4061/2011/276393. Int J Alzheimers Dis. 2011. PMID: 21423554 Free PMC article.
-
Rivastigmine modifies the α-secretase pathway and potentially early Alzheimer's disease.Transl Psychiatry. 2020 Feb 3;10(1):47. doi: 10.1038/s41398-020-0709-x. Transl Psychiatry. 2020. PMID: 32066688 Free PMC article.
-
Iron response elements (IREs)-mRNA of Alzheimer's amyloid precursor protein binding to iron regulatory protein (IRP1): a combined molecular docking and spectroscopic approach.Sci Rep. 2023 Mar 28;13(1):5073. doi: 10.1038/s41598-023-32073-x. Sci Rep. 2023. PMID: 36977734 Free PMC article.
References
-
- Rogers J. T., Randall J. D., Cahill C. M., Eder P. S., Huang X., Gunshin H., Leiter L., McPhee J., Sarang S. S., Utsuki T., Greig N. H., Lahiri D. K., Tanzi R. E., Bush A. I., Giordano T., Gullans S. R. (2002) J. Biol. Chem. 277, 45518–45528 - PubMed
-
- Wolfe M. S. (2006) Sci. Am. 294, 72–79 - PubMed
-
- Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002) Nature 416, 535–539 - PubMed
-
- Lee V. M., Giasson B. I., Trojanowski J. Q. (2004) Trends Neurosci. 27, 129–134 - PubMed
-
- Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., Selkoe D. J. (1999) Nature 398, 513–517 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

