Secreted heat shock protein 90alpha induces colorectal cancer cell invasion through CD91/LRP-1 and NF-kappaB-mediated integrin alphaV expression
- PMID: 20558745
- PMCID: PMC2919109
- DOI: 10.1074/jbc.M110.139345
Secreted heat shock protein 90alpha induces colorectal cancer cell invasion through CD91/LRP-1 and NF-kappaB-mediated integrin alphaV expression
Abstract
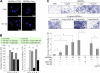
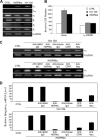
HCT-8 colon cancer cells secreted heat shock protein 90alpha (HSP90alpha) and had increased invasiveness upon serum starvation. The concentrated conditioned medium of serum-starved HCT-8 cells was able to stimulate the migration and invasion of non-serum-starved cells, which could be prevented by treatment with an anti-HSP90alpha antibody. Recombinant HSP90alpha (rHSP90alpha) also enhanced HCT-8 cell migration and invasion, suggesting a stimulatory role of secreted HSP90alpha in cancer malignancy. HSP90alpha binding to CD91alpha and Neu was evidenced by the proximity ligation assay, and rHSP90alpha-induced HCT-8 cell invasion could be suppressed by the addition of anti-CD91alpha or anti-Neu antibodies. Via CD91alpha and Neu, rHSP90alpha selectively induced integrin alpha(V) expression, and knockdown of integrin alpha(V) efficiently blocked rHSP90alpha-induced HCT-8 cell invasion. rHSP90alpha induced the activities of ERK, PI3K/Akt, and NF-kappaB p65, but only NF-kappaB activation was involved in HSP90alpha-induced integrin alpha(V) expression. Additionally, we investigated the serum levels of HSP90alpha and the expression status of tumor integrin alpha(V) mRNA in colorectal cancer patients. Serum HSP90alpha levels of colorectal cancer patients were significantly higher than those of normal volunteers (p < 0.001). Patients with higher serum HSP90alpha levels significantly exhibited elevated levels of integrin alpha(V) mRNA in tumor tissues as compared with adjacent non-tumor tissues (p < 0.001). Furthermore, tumor integrin alpha(V) overexpression was significantly correlated with TNM (Tumor, Node, Metastasis) staging (p = 0.001).
Figures





References
-
- Gething M. J., Sambrook J. (1992) Nature 355, 33–45 - PubMed
-
- Goetz M. P., Toft D. O., Ames M. M., Erlichman C. (2003) Ann. Oncol. 14, 1169–1176 - PubMed
-
- Sidera K., Samiotaki M., Yfanti E., Panayotou G., Patsavoudi E. (2004) J. Biol. Chem. 279, 45379–45388 - PubMed
-
- Becker B., Multhoff G., Farkas B., Wild P. J., Landthaler M., Stolz W., Vogt T. (2004) Exp. Dermatol. 13, 27–32 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

