miR-802 regulates human angiotensin II type 1 receptor expression in intestinal epithelial C2BBe1 cells
- PMID: 20558762
- PMCID: PMC2950689
- DOI: 10.1152/ajpgi.00120.2010
miR-802 regulates human angiotensin II type 1 receptor expression in intestinal epithelial C2BBe1 cells
Abstract
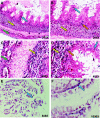
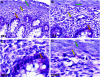
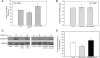
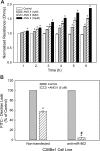
Studies have demonstrated that angiotensin II (Ang II) can regulate intestinal fluid and electrolyte transport and control intestinal wall muscular activity. Ang II is also a proinflammatory mediator that participates in inflammatory responses such as apoptosis, angiogenesis, and vascular remodeling; accumulating evidence suggests that this hormone may be involved in gastrointestinal (GI) inflammation and carcinogenesis. Ang II binds to two distinct G protein-coupled receptor subtypes, the AT(1)R and AT(2)R, which are widely expressed in the GI system. Together these studies suggest that Ang II-AT(1)R/-AT(2)R actions may play an important role in GI tract physiology and pathophysiology. Currently it is not known whether miRNAs can regulate the expression of the human AT(1)R (hAT(1)R) in the GI system. PCR and in situ hybridization experiments demonstrated that miR-802 was abundantly expressed in human colon and intestine. Luciferase reporter assays demonstrated that miR-802 could directly interact with the bioinformatics-predicted target site harbored within the 3'-untranslated region of the hAT(1)R mRNA. To validate that the levels of miR-802 were physiologically relevant in the GI system, we demonstrated that miR-802 "loss-of-function" experiments resulted in augmented hAT(1)R levels and enhanced Ang II-induced signaling in a human intestinal epithelial cell line. These results suggest that miR-802 can modulate the expression of the hAT(1)R in the GI tract and ultimately play a role in regulating the biological efficacy of Ang II in this system.
Figures


Similar articles
-
MicroRNA-155 regulates human angiotensin II type 1 receptor expression in fibroblasts.J Biol Chem. 2006 Jul 7;281(27):18277-84. doi: 10.1074/jbc.M601496200. Epub 2006 May 4. J Biol Chem. 2006. Retraction in: J Biol Chem. 2013 Feb 8;288(6):4226. doi: 10.1074/jbc.A112.601496. PMID: 16675453 Retracted.
-
The human angiotensin II type 1 receptor +1166 A/C polymorphism attenuates microRNA-155 binding.J Biol Chem. 2007 Aug 17;282(33):24262-9. doi: 10.1074/jbc.M701050200. Epub 2007 Jun 22. J Biol Chem. 2007. Retraction in: J Biol Chem. 2013 Feb 8;288(6):4227. doi: 10.1074/jbc.A112.701050. PMID: 17588946 Free PMC article. Retracted.
-
Small RNA sequencing reveals microRNAs that modulate angiotensin II effects in vascular smooth muscle cells.J Biol Chem. 2012 May 4;287(19):15672-83. doi: 10.1074/jbc.M111.322669. Epub 2012 Mar 19. J Biol Chem. 2012. PMID: 22431733 Free PMC article.
-
The angiotensin II type 2 receptor and the gastrointestinal tract.J Renin Angiotensin Aldosterone Syst. 2010 Mar;11(1):43-8. doi: 10.1177/1470320309347788. Epub 2009 Oct 27. J Renin Angiotensin Aldosterone Syst. 2010. PMID: 19861352 Review.
-
The renin-angiotensin system and the gastrointestinal mucosa.Acta Physiol (Oxf). 2011 Jan;201(1):157-67. doi: 10.1111/j.1748-1716.2010.02165.x. Acta Physiol (Oxf). 2011. PMID: 20626369 Review.
Cited by
-
Epigenetic processes during preeclampsia and effects on fetal development and chronic health.Clin Sci (Lond). 2021 Oct 15;135(19):2307-2327. doi: 10.1042/CS20190070. Clin Sci (Lond). 2021. PMID: 34643675 Free PMC article. Review.
-
miR-802 participates in the inflammatory process of inflammatory bowel disease by suppressing SOCS5.Biosci Rep. 2020 Apr 30;40(4):BSR20192257. doi: 10.1042/BSR20192257. Biosci Rep. 2020. Retraction in: Biosci Rep. 2024 Nov 27;44(11):BSR-2019-2257_RET. doi: 10.1042/BSR-2019-2257_RET. PMID: 32211804 Free PMC article. Retracted.
-
Screening out microRNAs and Their Molecular Pathways with a Potential Role in the Regulation of Parvovirus B19 Infection Through In Silico Analysis.Int J Mol Sci. 2025 May 23;26(11):5038. doi: 10.3390/ijms26115038. Int J Mol Sci. 2025. PMID: 40507847 Free PMC article.
-
Experimental procedures to identify and validate specific mRNA targets of miRNAs.EXCLI J. 2015 Jul 2;14:758-90. doi: 10.17179/excli2015-319. eCollection 2015. EXCLI J. 2015. PMID: 27047316 Free PMC article. Review.
-
Bioinformatic Investigation of Micro RNA-802 Target Genes, Protein Networks, and Its Potential Prognostic Value in Breast Cancer.Avicenna J Med Biotechnol. 2022 Apr-Jun;14(2):154-164. doi: 10.18502/ajmb.v14i2.8882. Avicenna J Med Biotechnol. 2022. PMID: 35633990 Free PMC article.
References
-
- Alexiou P, Maragkakis M, Papadopoulos GL, Reczko M, Hatzigeorgiou AG. Lost in translation: an assessment and perspective for computational microRNA target identification. Bioinformatics 25: 3049–3055, 2009 - PubMed
-
- Berk BC. Angiotensin type 2 receptor (AT2R): a challenging twin. Sci STKE 181: PE16, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

