Role of clock genes in gastrointestinal motility
- PMID: 20558764
- PMCID: PMC2950682
- DOI: 10.1152/ajpgi.00147.2010
Role of clock genes in gastrointestinal motility
Abstract
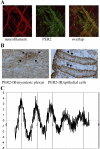
Biological rhythms coordinate the timing of our internal bodily functions. Colonic motility follows a rhythm as well: most people will have a bowel movement in the morning and rarely during the night. Recent work provides a potential mechanism for this observation: the mouse colon possesses a functional circadian clock as well as a subset of rhythmically expressed genes that may directly impact on colonic motility. Furthermore, measures of colonic motility such as the colonic tissue contractile response to acetylcholine, stool output, and intracolonic pressure changes vary as a function of the time of day, but these variations are attenuated in mice with disrupted clock function. These laboratory findings are supported by clinical observations. Gastrointestinal symptoms such as diarrhea or constipation are prevalent among shift workers and time-zone travelers, both of which are conditions associated with disruptions in biological rhythms. This review will discuss new insights into the role of clock genes in colonic motility and their potential clinical relevance.
Figures
Similar articles
-
Rhythmic changes in colonic motility are regulated by period genes.Am J Physiol Gastrointest Liver Physiol. 2010 Feb;298(2):G143-50. doi: 10.1152/ajpgi.00402.2009. Epub 2009 Nov 19. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 19926812 Free PMC article.
-
Role of biological rhythms in gastrointestinal health and disease.Rev Endocr Metab Disord. 2009 Dec;10(4):293-300. doi: 10.1007/s11154-009-9119-3. Rev Endocr Metab Disord. 2009. PMID: 19798581 Review.
-
Disruption of Circadian Rhythms and Gut Motility: An Overview of Underlying Mechanisms and Associated Pathologies.J Clin Gastroenterol. 2020 May/Jun;54(5):405-414. doi: 10.1097/MCG.0000000000001333. J Clin Gastroenterol. 2020. PMID: 32134798 Free PMC article. Review.
-
Biologic clocks and the gut.Curr Gastroenterol Rep. 2006 Oct;8(5):353-9. doi: 10.1007/s11894-006-0019-3. Curr Gastroenterol Rep. 2006. PMID: 16968601 Review.
-
[Clock genes and circadian rhythm of blood pressure].Nihon Rinsho. 2014 Aug;72(8):1354-60. Nihon Rinsho. 2014. PMID: 25167735 Review. Japanese.
Cited by
-
Entrainment of the Circadian Clock of the Enteric Bacterium Klebsiella aerogenes by Temperature Cycles.iScience. 2019 Sep 27;19:1202-1213. doi: 10.1016/j.isci.2019.09.007. Epub 2019 Sep 10. iScience. 2019. PMID: 31551197 Free PMC article.
-
Association between night work and dyslipidemia in South Korean men and women: a cross-sectional study.Lipids Health Dis. 2019 Mar 28;18(1):75. doi: 10.1186/s12944-019-1020-9. Lipids Health Dis. 2019. PMID: 30922333 Free PMC article.
-
Effects of Electroacupuncture on the Daily Rhythmicity of Intestinal Movement and Circadian Rhythmicity of Colonic Per2 Expression in Rats with Spinal Cord Injury.Biomed Res Int. 2016;2016:9860281. doi: 10.1155/2016/9860281. Epub 2016 Nov 24. Biomed Res Int. 2016. PMID: 27999821 Free PMC article.
-
Influence of Chronotype and Theobromine on the 24-h Variation in Peak Expiratory Flow Rate in Healthy Adults.J Circadian Rhythms. 2025 Apr 3;23:2. doi: 10.5334/jcr.242. eCollection 2025. J Circadian Rhythms. 2025. PMID: 40182321 Free PMC article.
-
Circadian rhythms in the pathogenesis of gastrointestinal diseases.World J Gastroenterol. 2018 Oct 14;24(38):4297-4303. doi: 10.3748/wjg.v24.i38.4297. World J Gastroenterol. 2018. PMID: 30344415 Free PMC article.
References
-
- Auwerda JJ, Bac DJ, Schouten WR. Circadian rhythm of rectal motor complexes. Dis Colon Rectum 44: 1328–1332, 2001 - PubMed
-
- Bailey MJ, Beremand PD, Hammer R, Bell-Pedersen D, Thomas TL, Cassone VM. Transcriptional profiling of the chick pineal gland, a photoreceptive circadian oscillator and pacemaker. Mol Endocrinol 17: 2084–2095, 2003 - PubMed
-
- Bostwick J, Nguyen D, Cornelissen G, Halberg F, Hoogerwerf WA. Effects of acute and chronic STZ-induced diabetes on clock gene expression and feeding in the gastrointestinal tract. Mol Cell Biochem 338: 203–213, 2010 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

