An update on sphingosine-1-phosphate and other sphingolipid mediators
- PMID: 20559316
- PMCID: PMC3001344
- DOI: 10.1038/nchembio.392
An update on sphingosine-1-phosphate and other sphingolipid mediators
Erratum in
- Nat Chem Biol. 2010 Sep;6(9):689
Abstract
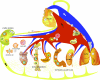
Sphingolipids comprise a complex family of naturally occurring molecules that are enriched in lipid rafts and contribute to their unique biochemical properties. Membrane sphingolipids also serve as a reservoir for bioactive metabolites including sphingosine, ceramide, sphingosine-1-phosphate and ceramide-1-phosphate. Among these, sphingosine-1-phosphate has emerged as a central regulator of mammalian biology. Sphingosine-1-phosphate is essential for mammalian brain and cardiac development and for maturation of the systemic circulatory system and lymphatics. In addition, sphingosine-1-phosphate contributes to trafficking and effector functions of lymphocytes and other hematopoietic cells and protects against various forms of tissue injury. However, sphingosine-1-phosphate is also an oncogenic lipid that promotes tumor growth and progression. Recent preclinical and clinical investigations using pharmacological agents that target sphingosine-1-phosphate, its receptors and the enzymes required for its biosynthesis and degradation demonstrate the promise and potential risks of modulating sphingosine-1-phosphate signaling in treatment strategies for autoimmunity, cancer, cardiovascular disease and other pathological conditions.
Figures


References
-
- Holthuis JC, Pomorski T, Raggers RJ, Sprong H, Van Meer G. The organizing potential of sphingolipids in intracellular membrane transport. Physiol Rev. 2001;81:1689–723. - PubMed
-
- Merrill AHJ, Sandhoff K. Sphingolipids: metabolism and cell signaling. In: Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins and Membranes. Chapter 14; 2002. pp. 373–407.
-
- Mandala S, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–9. - PubMed
-
- Brinkmann V, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

