Tumor cells engineered to codisplay on their surface 4-1BBL and LIGHT costimulatory proteins as a novel vaccine approach for cancer immunotherapy
- PMID: 20559332
- PMCID: PMC2941532
- DOI: 10.1038/cgt.2010.29
Tumor cells engineered to codisplay on their surface 4-1BBL and LIGHT costimulatory proteins as a novel vaccine approach for cancer immunotherapy
Abstract
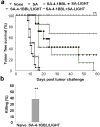
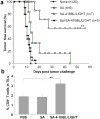
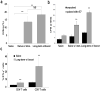
Primary tumor cells genetically modified to express a collection of immunological ligands on their surface may have the utility as therapeutic autologous cancer vaccines. However, genetic modification of primary tumor cells is not only cost, labor and time intensive, but also has safety repercussions. As an alternative, we developed the ProtEx technology that involves generation of immunological ligands with core streptavidin (SA) and their display on biotinylated cells in a rapid and efficient manner. We herein demonstrate that TC-1 tumor cells can be rapidly and efficiently engineered to codisplay on their surface two costimulatory proteins, SA-4-1BBL and SA-LIGHT, simultaneously. Vaccination with irradiated TC-1 cells codisplaying both chimeric proteins showed 100% efficacy in a prophylactic and >55% efficacy in a therapeutic tumor setting. In contrast, vaccination with TC-1 cells engineered with either protein alone showed significantly reduced efficacy in the prophylactic setting. Vaccine efficacy was associated with the generation of primary and memory T-cell and antibody responses against the tumor without detectable signs of autoimmunity. Engineering tumor cells in a rapid and effective manner to simultaneously display on their surface a collection of immunostimulatory proteins with additive/synergistic functions presents a novel alternative approach to gene therapy with considerable potential for cancer immunotherapy.
Conflict of interest statement
Figures



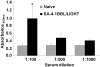
Similar articles
-
SA-4-1BBL as the immunomodulatory component of a HPV-16 E7 protein based vaccine shows robust therapeutic efficacy in a mouse cervical cancer model.Vaccine. 2010 Aug 16;28(36):5794-802. doi: 10.1016/j.vaccine.2010.06.073. Epub 2010 Jul 4. Vaccine. 2010. PMID: 20603135 Free PMC article.
-
Provision of 4-1BB ligand enhances effector and memory CTL responses generated by immunization with dendritic cells expressing a human tumor-associated antigen.J Immunol. 2003 Mar 15;170(6):2912-22. doi: 10.4049/jimmunol.170.6.2912. J Immunol. 2003. PMID: 12626542
-
4-1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines.Cancer Res. 2010 May 15;70(10):3945-54. doi: 10.1158/0008-5472.CAN-09-4480. Epub 2010 Apr 20. Cancer Res. 2010. PMID: 20406989 Free PMC article.
-
SA-4-1BBL as a novel adjuvant for the development of therapeutic cancer vaccines.Expert Rev Vaccines. 2014 Mar;13(3):387-98. doi: 10.1586/14760584.2014.880340. Expert Rev Vaccines. 2014. PMID: 24521311 Free PMC article. Review.
-
ProtEx technology for the generation of novel therapeutic cancer vaccines.Exp Mol Pathol. 2009 Jun;86(3):198-207. doi: 10.1016/j.yexmp.2009.01.010. Epub 2009 Jan 31. Exp Mol Pathol. 2009. PMID: 19454266 Free PMC article. Review.
Cited by
-
Targeting of the tumor necrosis factor receptor superfamily for cancer immunotherapy.ISRN Oncol. 2013 Jun 11;2013:371854. doi: 10.1155/2013/371854. Print 2013. ISRN Oncol. 2013. PMID: 23840967 Free PMC article.
-
Tumor-derived granulocyte colony-stimulating factor diminishes efficacy of breast tumor cell vaccines.Breast Cancer Res. 2018 Oct 22;20(1):126. doi: 10.1186/s13058-018-1054-3. Breast Cancer Res. 2018. PMID: 30348199 Free PMC article.
-
A fusion protein between streptavidin and the endogenous TLR4 ligand EDA targets biotinylated antigens to dendritic cells and induces T cell responses in vivo.Biomed Res Int. 2013;2013:864720. doi: 10.1155/2013/864720. Epub 2013 Sep 5. Biomed Res Int. 2013. PMID: 24093105 Free PMC article.
-
4-1BB Signaling in Conventional T Cells Drives IL-2 Production That Overcomes CD4+CD25+FoxP3+ T Regulatory Cell Suppression.PLoS One. 2016 Apr 6;11(4):e0153088. doi: 10.1371/journal.pone.0153088. eCollection 2016. PLoS One. 2016. PMID: 27049955 Free PMC article.
-
SA-4-1BBL and monophosphoryl lipid A constitute an efficacious combination adjuvant for cancer vaccines.Cancer Res. 2014 Nov 15;74(22):6441-51. doi: 10.1158/0008-5472.CAN-14-1768-A. Epub 2014 Sep 24. Cancer Res. 2014. PMID: 25252915 Free PMC article.
References
-
- Anderson RC, Anderson DE, Elder JB, et al. Lack of B7 expression, not human leukocyte antigen expression, facilitates immune evasion by human malignant gliomas. Neurosurgery. 2007;60:1129–36. - PubMed
-
- van de Corp, Falkenburg JH, Kester MG, Willemze R, Kluin-Nelemans JC. Impaired expression of CD28 on T cells in hairy cell leukemia. Clin Immunol. 1999;93:256–62. - PubMed
-
- Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

