Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development
- PMID: 20559386
- PMCID: PMC2909826
- DOI: 10.1038/nature09079
Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development
Abstract
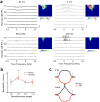
Functional receptive fields of neurons in sensory cortices undergo progressive refinement during development. Such refinement may be attributed to the pruning of non-optimal excitatory inputs, reshaping of the excitatory tuning profile through modifying the strengths of individual inputs, or strengthening of cortical inhibition. These models have not been directly tested because of the technical difficulties in assaying the spatiotemporal patterns of functional synaptic inputs during development. Here we apply in vivo whole-cell voltage-clamp recordings to the recipient layer 4 neurons in the rat primary auditory cortex (A1) to determine the developmental changes in the frequency-intensity tonal receptive fields (TRFs) of their excitatory and inhibitory inputs. Surprisingly, we observe co-tuned excitation and inhibition immediately after the onset of hearing, suggesting that a tripartite thalamocortical circuit with relatively strong feedforward inhibition is formed independently of auditory experience. The frequency ranges of tone-driven excitatory and inhibitory inputs first expand within a few days of the onset of hearing and then persist into adulthood. The latter phase is accompanied by a sharpening of the excitatory but not inhibitory frequency tuning profile, which results in relatively broader inhibitory tuning in adult A1 neurons. Thus the development of cortical synaptic TRFs after the onset of hearing is marked by a slight breakdown of previously formed excitation-inhibition balance. Our results suggest that functional refinement of cortical TRFs does not require a selective pruning of inputs, but may depend more on a fine adjustment of excitatory input strengths.
Figures



Comment in
-
Sensory systems: A balancing act.Nat Rev Neurosci. 2010 Aug;11(8):536-7. doi: 10.1038/nrn2885. Nat Rev Neurosci. 2010. PMID: 20672428 No abstract available.
References
-
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–8. - PubMed
-
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–20. - PubMed
-
- Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4:1123–30. - PubMed
-
- Inan M, Crair MC. Development of cortical maps: perspectives from the barrel cortex. Neuroscientist. 2007;13:49–61. - PubMed
-
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–88. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

