Targeting the Lipid Metabolic Pathways for the Treatment of Malaria
- PMID: 20559451
- PMCID: PMC2886290
- DOI: 10.1002/ddr.20347
Targeting the Lipid Metabolic Pathways for the Treatment of Malaria
Abstract
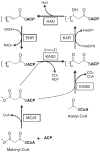
The control and eventual eradication of human malaria is considered one of the most important global public health goals of the 21st Century. Malaria, caused by intraerythrocytic protozoan parasites of the genus Plasmodium, is by far the most lethal and among the most prevalent of the infectious diseases. Four species of Plasmodium (P. falciparum, P. malariae, P. ovale, and P. vivax) are known to be infectious to humans, and more recent cases of infection due to P. knowlesi also have been reported. These species cause approximately 300 million annual cases of clinical malaria resulting in around one million deaths mostly caused by P. falciparum. The rapid emergence of drug-resistant Plasmodium strains has severely reduced the potency of medicines commonly used to treat and block the transmission of malaria and threatens the effectiveness of combination therapy in the field. New drugs that target important parasite functions, which are not the target of current antimalarial drugs, and have the potential to act against multi-drug-resistant Plasmodium strains are urgently needed. Recent studies in P. falciparum have unraveled new metabolic pathways for the synthesis of the parasite phospholipids and fatty acids. The present review summarizes our current understanding of these pathways in Plasmodium development and pathogenesis, and provides an update on the efforts underway to characterize their importance using genetic means and to develop antimalarial therapies targeting lipid metabolic pathways.
Figures


References
-
- Ancelin ML, Vial HJ, Philippot JR. Inhibitors of choline transport into Plasmodium-infected erythrocytes are effective antiplasmodial compounds in vitro. Biochem Pharmacol. 1985;34:4068–4071. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

