The N-terminal 81-aa fragment is critical for UT-A1 urea transporter bioactivity
- PMID: 20559454
- PMCID: PMC2886301
- DOI: 10.2174/1875044301003010034
The N-terminal 81-aa fragment is critical for UT-A1 urea transporter bioactivity
Abstract
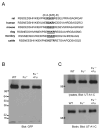
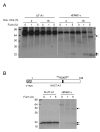
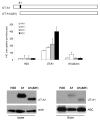
The serine protease, furin, is involved in the activation of a number of proteins most notably epithelial sodium channels (ENaC). The urea transporter UT-A1, located in the kidney inner medullary collecting duct (IMCD), is important for urine concentrating ability. UT-A1's amino acid sequence has a consensus furin cleavage site (RSKR) in the N-terminal region. Despite the putative cleavage site, we find that UT-A1, either from the cytosolic or cell surface pool, is not cleaved by furin in CHO cells. This result was further confirmed by an inability of furin to cleave in vitro an (35)S-labeled UT-A1 or the 126 N-terminal UT-A1 fragment. Functionally, mutation of the furin site (R78A, R81A) does not affect UT-A1 urea transport activity. However, deletion of the 81-aa N-terminal portion does not affect UT-A1 cell surface trafficking, but seriously impair UT-A1 urea transport activity. Our results indicate that UT-A1 maturation and activation does not require furin-dependent cleavage. The N-terminal 81-aa fragment is required for proper UT-A1 urea transport activity, but its effect is not through changing UT-A1 membrane trafficking.
Figures

Similar articles
-
Lack of urea transporters, UT-A1 and UT-A3, increases nitric oxide accumulation to dampen medullary sodium reabsorption through ENaC.Am J Physiol Renal Physiol. 2019 Mar 1;316(3):F539-F549. doi: 10.1152/ajprenal.00166.2018. Epub 2018 Dec 12. Am J Physiol Renal Physiol. 2019. PMID: 30539654 Free PMC article.
-
Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct.Proc Natl Acad Sci U S A. 2004 May 11;101(19):7469-74. doi: 10.1073/pnas.0401704101. Epub 2004 May 3. Proc Natl Acad Sci U S A. 2004. PMID: 15123796 Free PMC article.
-
Regulation of urea transporter proteins in kidney and liver.Mt Sinai J Med. 2000 Mar;67(2):112-9. Mt Sinai J Med. 2000. PMID: 10747366 Review.
-
Activation of protein kinase Cα increases phosphorylation of the UT-A1 urea transporter at serine 494 in the inner medullary collecting duct.Am J Physiol Cell Physiol. 2015 Nov 1;309(9):C608-15. doi: 10.1152/ajpcell.00171.2014. Epub 2015 Sep 2. Am J Physiol Cell Physiol. 2015. PMID: 26333598 Free PMC article.
-
New advances in urea transporter UT-A1 membrane trafficking.Int J Mol Sci. 2013 May 21;14(5):10674-82. doi: 10.3390/ijms140510674. Int J Mol Sci. 2013. PMID: 23698785 Free PMC article. Review.
Cited by
-
Modulation of kidney urea transporter UT-A3 activity by alpha2,6-sialylation.Pflugers Arch. 2016 Jul;468(7):1161-1170. doi: 10.1007/s00424-016-1802-0. Epub 2016 Mar 14. Pflugers Arch. 2016. PMID: 26972907 Free PMC article.
-
Glycoforms of UT-A3 urea transporter with poly-N-acetyllactosamine glycosylation have enhanced transport activity.Am J Physiol Renal Physiol. 2012 Jul 15;303(2):F201-8. doi: 10.1152/ajprenal.00140.2012. Epub 2012 Apr 25. Am J Physiol Renal Physiol. 2012. PMID: 22535801 Free PMC article.
-
The emerging physiological roles of the SLC14A family of urea transporters.Br J Pharmacol. 2011 Dec;164(7):1780-92. doi: 10.1111/j.1476-5381.2011.01377.x. Br J Pharmacol. 2011. PMID: 21449978 Free PMC article. Review.
-
Mature N-linked glycans facilitate UT-A1 urea transporter lipid raft compartmentalization.FASEB J. 2011 Dec;25(12):4531-9. doi: 10.1096/fj.11-185991. Epub 2011 Sep 29. FASEB J. 2011. PMID: 21965602 Free PMC article.
References
-
- Sands JM. Renal urea transporters. Curr Opin Nephrol Hypertens. 2004;13:525–32. - PubMed
-
- Karakashian A, Timmer RT, Klein JD, Gunn RB, Sands JM, Bagnasco SM. Cloning and characterization of two new isoforms of the rat kidney urea transporter: UT-A3 and UT-A4. J Am Soc Nephrol. 1999;10:230–7. - PubMed
-
- Bradford AD, Terris JM, Ecelbarger CA, Klein JD, Sands JM, Chou CL, Knepper MA. 97- and 117-kDa forms of collecting duct urea transporter UT-A1 are due to different states of glycosylation. Am J Physiol Renal Physiol. 2001;281:F133–43. - PubMed
-
- Chen G, Fröhlich O, Yang Y, Klein JD, Sands JM. Loss of N-linked glycosylation reduces urea transporter UT-A1 response to vasopressin. J Biol Chem. 2006;281:27436–42. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
