Application of spectroscopic methods for structural analysis of chitin and chitosan
- PMID: 20559489
- PMCID: PMC2885081
- DOI: 10.3390/md8051567
Application of spectroscopic methods for structural analysis of chitin and chitosan
Abstract
Chitin, the second most important natural polymer in the world, and its N-deacetylated derivative chitosan, have been identified as versatile biopolymers for a broad range of applications in medicine, agriculture and the food industry. Two of the main reasons for this are firstly the unique chemical, physicochemical and biological properties of chitin and chitosan, and secondly the unlimited supply of raw materials for their production. These polymers exhibit widely differing physicochemical properties depending on the chitin source and the conditions of chitosan production. The presence of reactive functional groups as well as the polysaccharide nature of these biopolymers enables them to undergo diverse chemical modifications. A complete chemical and physicochemical characterization of chitin, chitosan and their derivatives is not possible without using spectroscopic techniques. This review focuses on the application of spectroscopic methods for the structural analysis of these compounds.
Keywords: chemical modification; chitin; chitosan; physicochemical parameters; structural analysis using spectroscopic techniques.
Figures
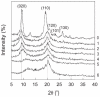
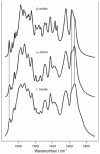
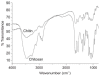

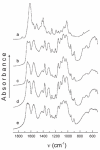
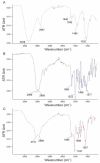
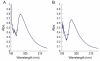
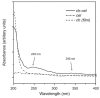
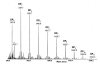
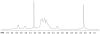
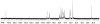
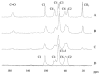
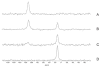


Similar articles
-
Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket.Biomed Mater. 2018 Jan 30;13(2):025009. doi: 10.1088/1748-605X/aa9dde. Biomed Mater. 2018. PMID: 29182521
-
Investigation on Mecynorhina torquata Drury, 1782 (Coleoptera, Cetoniidae, Goliathini) cuticle: Surface properties, chitin and chitosan extraction.Int J Biol Macromol. 2020 Dec 1;164:1164-1173. doi: 10.1016/j.ijbiomac.2020.07.155. Epub 2020 Jul 20. Int J Biol Macromol. 2020. PMID: 32702421
-
Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers.Appl Biochem Biotechnol. 2017 Apr;181(4):1314-1337. doi: 10.1007/s12010-016-2286-2. Epub 2016 Oct 27. Appl Biochem Biotechnol. 2017. PMID: 27787767 Review.
-
Preparative methods of phosphorylated chitin and chitosan--an overview.Int J Biol Macromol. 2008 Oct 1;43(3):221-5. doi: 10.1016/j.ijbiomac.2008.07.004. Epub 2008 Jul 9. Int J Biol Macromol. 2008. PMID: 18657571 Review.
-
Extraction and physicochemical characterization of chitin and chitosan from Zophobas morio larvae in varying sodium hydroxide concentration.Int J Biol Macromol. 2018 Mar;108:135-142. doi: 10.1016/j.ijbiomac.2017.11.138. Epub 2017 Nov 22. Int J Biol Macromol. 2018. PMID: 29175166
Cited by
-
Chitosan as a Functional Carrier for the Local Delivery Anti-Inflammatory Systems Containing Scutellariae baicalensis radix Extract.Pharmaceutics. 2022 Oct 10;14(10):2148. doi: 10.3390/pharmaceutics14102148. Pharmaceutics. 2022. PMID: 36297583 Free PMC article.
-
Bio-Functionalized Chitosan for Bone Tissue Engineering.Int J Mol Sci. 2021 May 31;22(11):5916. doi: 10.3390/ijms22115916. Int J Mol Sci. 2021. PMID: 34072888 Free PMC article.
-
Low Temperature Dissolution of Yeast Chitin-Glucan Complex and Characterization of the Regenerated Polymer.Bioengineering (Basel). 2020 Mar 14;7(1):28. doi: 10.3390/bioengineering7010028. Bioengineering (Basel). 2020. PMID: 32183337 Free PMC article.
-
Chitosan-coated magnetic nanoparticles prepared in one step by reverse microemulsion precipitation.Int J Mol Sci. 2013 Sep 27;14(10):19636-50. doi: 10.3390/ijms141019636. Int J Mol Sci. 2013. PMID: 24084716 Free PMC article.
-
Fungi-based treatment of brewery wastewater-biomass production and nutrient reduction.Appl Microbiol Biotechnol. 2017 Jun;101(11):4791-4798. doi: 10.1007/s00253-017-8185-9. Epub 2017 Feb 17. Appl Microbiol Biotechnol. 2017. PMID: 28213731 Free PMC article.
References
-
- Muzzarelli RAA, Jeuniaux C, Gooday GW. Chitin in Nature and Technology. Plenum Publishing Corporation; New York, NY, USA: 1986.
-
- Rinaudo M. Chitin and chitosan: Properties and application. Prog Polym Sci. 2006;31:603–632.
-
- Roberts GAF. Chitin Chemistry. 1st ed. MacMillan; London, UK: 1992.
-
- Austin PR. Chitin solutions and purification of chitin. Methods Enzymol. 1988;161:403–407.
-
- Kurita K. Controlled functionalization of the polysaccharide chitin. Progr Polym Sci. 2001;26:1921–1971.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
