Spatial and genetic epidemiology of hookworm in a rural community in Uganda
- PMID: 20559556
- PMCID: PMC2886101
- DOI: 10.1371/journal.pntd.0000713
Spatial and genetic epidemiology of hookworm in a rural community in Uganda
Abstract
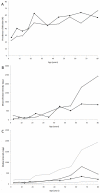
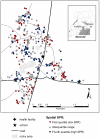
There are remarkably few contemporary, population-based studies of intestinal nematode infection for sub-Saharan Africa. This paper presents a comprehensive epidemiological analysis of hookworm infection intensity in a rural Ugandan community. Demographic, kinship, socioeconomic and environmental data were collected for 1,803 individuals aged six months to 85 years in 341 households in a cross-sectional community survey. Hookworm infection was assessed by faecal egg count. Spatial variation in the intensity of infection was assessed using a Bayesian negative binomial spatial regression model and the proportion of variation explained by host additive genetics (heritability) and common domestic environment was estimated using genetic variance component analysis. Overall, the prevalence of hookworm was 39.3%, with the majority of infections (87.7%) of light intensity (<or=1000 eggs per gram faeces). Intensity was higher among older individuals and was associated with treatment history with anthelmintics, walking barefoot outside the home, living in a household with a mud floor and education level of the household head. Infection intensity also exhibited significant household and spatial clustering: the range of spatial correlation was estimated to be 82 m and was reduced by a half over a distance of 19 m. Heritability of hookworm egg count was 11.2%, whilst the percentage of variance explained by unidentified domestic effects was 17.8%. In conclusion, we suggest that host genetic relatedness is not a major determinant of infection intensity in this community, with exposure-related factors playing a greater role.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Behnke JM, De Clercq D, Sacko M, Gilbert FS, Ouattara DB, et al. The epidemiology of human hookworm infections in the southern region of Mali. Trop Med Int Health. 2000;5:343–354. - PubMed
-
- Palmer DR, Bundy DAP. Epidemiology of human hookworm and Ascaris lumbricoides infestations in rural Gambia. East Afr Med J. 1995;72:527–530. - PubMed
-
- Udonsi JK. Studies on the co-occurrence of two species of human hookworm in a riverine community in Nigeria. Tropenmed Parasitol. 1984;35:37–40. - PubMed
-
- Udonsi JK. Necator americanus infection: a cross-sectional study of a rural community in relation to some clinical symptoms. Ann Trop Med Parasitol. 1984;78:443–444. - PubMed
-
- Chandiwana SK, Bradley M, Chombo F. Hookworm and roundworm infections in farm-worker communities in the large-scale agricultural sector in Zimbabwe. J Trop Med Hyg. 1989;92:338–344. - PubMed

