Assessment of the potential role of muscle spindle mechanoreceptor afferents in chronic muscle pain in the rat masseter muscle
- PMID: 20559566
- PMCID: PMC2886111
- DOI: 10.1371/journal.pone.0011131
Assessment of the potential role of muscle spindle mechanoreceptor afferents in chronic muscle pain in the rat masseter muscle
Abstract
Background: The phenotype of large diameter sensory afferent neurons changes in several models of neuropathic pain. We asked if similar changes also occur in "functional" pain syndromes.
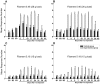
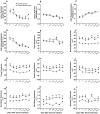
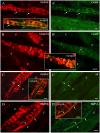
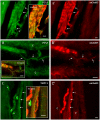
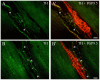
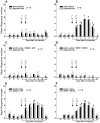
Methodology/principal findings: Acidic saline (AS, pH 4.0) injections into the masseter muscle were used to induce persistent myalgia. Controls received saline at pH 7.2. Nocifensive responses of Experimental rats to applications of Von Frey Filaments to the masseters were above control levels 1-38 days post-injection. This effect was bilateral. Expression of c-Fos in the Trigeminal Mesencephalic Nucleus (NVmes), which contains the somata of masseter muscle spindle afferents (MSA), was above baseline levels 1 and 4 days after AS. The resting membrane potentials of neurons exposed to AS (n = 167) were hyperpolarized when compared to their control counterparts (n = 141), as were their thresholds for firing, high frequency membrane oscillations (HFMO), bursting, inward and outward rectification. The amplitude of HFMO was increased and spontaneous ectopic firing occurred in 10% of acid-exposed neurons, but never in Controls. These changes appeared within the same time frame as the observed nocifensive behaviour. Ectopic action potentials can travel centrally, but also antidromically to the peripheral terminals of MSA where they could cause neurotransmitter release and activation of adjacent fibre terminals. Using immunohistochemistry, we confirmed that annulospiral endings of masseter MSA express the glutamate vesicular transporter VGLUT1, indicating that they can release glutamate. Many capsules also contained fine fibers that were labelled by markers associated with nociceptors (calcitonin gene-related peptide, Substance P, P2X3 receptors and TRPV1 receptors) and that expressed the metabotropic glutamate receptor, mGluR5. Antagonists of glutamatergic receptors given together with the 2(nd) injection of AS prevented the hypersensitivity observed bilaterally but were ineffective if given contralaterally.
Conclusions/significance: Low pH leads to changes in several electrical properties of MSA, including initiation of ectopic action potentials which could propagate centrally but could also invade the peripheral endings causing glutamate release and activation of nearby nociceptors within the spindle capsule. This peripheral drive could contribute both to the transition to, and maintenance of, persistent muscle pain as seen in some "functional" pain syndromes.
Conflict of interest statement
Figures





References
-
- Nordin M, Nystrom B, Wallin U, Hagbarth KE. Ectopic sensory discharges and paresthesiae in patients with disorders of peripheral nerves, dorsal roots and dorsal columns. Pain. 1984;20:231–245. - PubMed
-
- Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–194. - PubMed
-
- Xie Y, Zhang J, Petersen M, LaMotte RH. Functional changes in dorsal root ganglion cells after chronic nerve constriction in the rat. J Neurophysiol. 1995;73:1811–1820. - PubMed
-
- Sukhotinsky I, Ben-Dor E, Raber P, Devor M. Key role of the dorsal root ganglion in neuropathic tactile hypersensibility. Eur J Pain. 2004;8:135–143. - PubMed
-
- Tsuboi Y, Takeda M, Tanimoto T, Ikeda M, et al. Alteration of the second branch of the trigeminal nerve activity following inferior alveolar nerve transection in rats. Pain. 2004;111:323–334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

