Requirements for leukocyte transmigration via the transmembrane chemokine CX3CL1
- PMID: 20559678
- PMCID: PMC11115548
- DOI: 10.1007/s00018-010-0433-4
Requirements for leukocyte transmigration via the transmembrane chemokine CX3CL1
Abstract
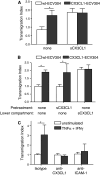
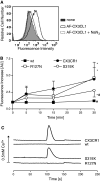
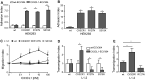
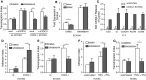
The surface-expressed transmembrane CX3C chemokine ligand 1 (CX3CL1/fractalkine) induces firm adhesion of leukocytes expressing its receptor CX3CR1. After shedding by the disintegrins and metalloproteinases (ADAM) 10 and 17, CX3CL1 also acts as soluble leukocyte chemoattractant. Here, we demonstrate that transmembrane CX3CL1 expressed on both endothelial and epithelial cells induces leukocyte transmigration. To investigate the underlying mechanism, we generated CX3CR1 variants lacking the intracellular aspartate-arginine-tyrosine (DRY) motif or the intracellular C-terminus which led to a defect in intracellular calcium response and impaired ligand uptake, respectively. While both variants effectively mediated firm cell adhesion, they failed to induce transmigration and rather mediated retention of leukocytes on the CX3CL1-expressing cell layer. Targeting of ADAM10 led to increased adhesion but reduced transmigration in response to transmembrane CX3CL1, while transmigration towards soluble CX3CL1 was not affected. Thus, transmembrane CX3CL1 mediates leukocyte transmigration via the DRY motif and C-terminus of CX3CR1 and the activity of ADAM10.
Figures


References
-
- McDermott DH, Fong AM, Yang Q, Sechler JM, Cupples LA, Merrell MN, Wilson PW, D’Agostino RB, O’Donnell CJ, Patel DD, Murphy PM. Chemokine receptor mutant CX3CR1–M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J Clin Invest. 2003;111:1241–1250. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

