Microneedle-based transcutaneous immunisation in mice with N-trimethyl chitosan adjuvanted diphtheria toxoid formulations
- PMID: 20559701
- PMCID: PMC2920068
- DOI: 10.1007/s11095-010-0182-y
Microneedle-based transcutaneous immunisation in mice with N-trimethyl chitosan adjuvanted diphtheria toxoid formulations
Abstract
Purpose: The purpose of this study was to gain insight into the delivery and immunogenicity of N-trimethyl chitosan (TMC) adjuvanted diphtheria toxoid (DT) formulations applied transcutaneously with microneedles.
Methods: Mice were vaccinated with DT-loaded TMC nanoparticles, a solution of TMC and DT (TMC/DT) or DT alone. The formulations were applied onto the skin before or after microneedle treatment with two different 300-microm-long microneedle arrays and also injected intradermally (ID). As a positive control, alum-adjuvanted DT (DT-alum) was injected subcutaneously (SC). Ex vivo confocal microscopy studies were performed with rhodamine-labelled TMC.
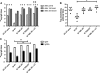
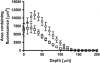
Results: Independent of the microneedle array used and the sequence of microneedle treatment and vaccine application, transcutaneous immunisation with the TMC/DT mixture elicited 8-fold higher IgG titres compared to the TMC nanoparticles or DT solution. The toxin-neutralising antibody titres from this group were similar to those elicited by SC DT-alum. After ID immunisation, both TMC-containing formulations induced enhanced titres compared to a DT solution. Confocal microscopy studies revealed that transport of the TMC nanoparticles across the microneedle conduits was limited compared to a TMC solution.
Conclusions: In conclusion, TMC has an adjuvant function in transcutaneous immunisation with microneedles, but only if applied in a solution.
Figures





References
-
- Immunization safety. www.who.int/immunization_safety/safe_injections/en/.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

