Targeting isoprenoid biosynthesis for drug discovery: bench to bedside
- PMID: 20560544
- PMCID: PMC2943567
- DOI: 10.1021/ar100026v
Targeting isoprenoid biosynthesis for drug discovery: bench to bedside
Abstract
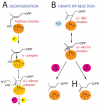
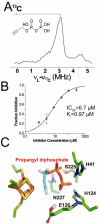
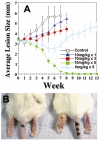
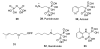
The isoprenoid biosynthesis pathways produce the largest class of small molecules in Nature: isoprenoids (also called terpenoids). Not surprisingly then, isoprenoid biosynthesis is a target for drug discovery, and many drugs--such as Lipitor (used to lower cholesterol), Fosamax (used to treat osteoporosis), and many anti-infectives--target isoprenoid biosynthesis. However, drug resistance in malaria, tuberculosis, and staph infections is rising, cheap and effective drugs for the neglected tropical diseases are lacking, and progress in the development of anticancer drugs is relatively slow. Isoprenoid biosynthesis is thus an attractive target, and in this Account, I describe developments in four areas, using in each case knowledge derived from one area of chemistry to guide the development of inhibitors (or drug leads) in another, seemingly unrelated, area. First, I describe mechanistic studies of the enzyme IspH, which is present in malaria parasites and most pathogenic bacteria, but not in humans. IspH is a 4Fe-4S protein and produces the five-carbon (C5) isoprenoids IPP (isopentenyl diphosphate) and DMAPP (dimethylallyl diphosphate) from HMBPP (E-1-hydroxy-2-methyl-but-2-enyl-4-diphosphate) via a 2H(+)/2e(-) reduction (of an allyl alcohol to an alkene). The mechanism is unusual in that it involves organometallic species: "metallacycles" (η(2)-alkenes) and η(1)/η(3)-allyls. These observations lead to novel alkyne inhibitors, which also form metallacycles. Second, I describe structure-function-inhibition studies of FPP synthase, the macromolecule that condenses IPP and DMAPP to the sesquiterpene farnesyl diphosphate (FPP) in a "head-to-tail" manner. This enzyme uses a carbocation mechanism and is potently inhibited by bone resorption drugs (bisphosphonates), which I show are also antiparasitic agents that block sterol biosynthesis in protozoa. Moreover, "lipophilic" bisphosphonates inhibit protein prenylation and invasiveness in tumor cells, in addition to activating γδ T-cells to kill tumor cells, and are important new leads in oncology. Third, I describe structural and inhibition studies of a "head-to-head" triterpene synthase, dehydrosqualene synthase (CrtM), from Staphylococcus aureus. CrtM catalyzes the first committed step in biosynthesis of the carotenoid virulence factor staphyloxanthin: the condensation of two FPP molecules to produce a cyclopropane (presqualene diphosphate). The structure of CrtM is similar to that of human squalene synthase (SQS), and some SQS inhibitors (originally developed as cholesterol-lowering drugs) block staphyloxanthin biosynthesis. Treated bacteria are white and nonvirulent (because they lack the carotenoid shield that protects them from reactive oxygen species produced by neutrophils), rendering them susceptible to innate immune system clearance--a new therapeutic approach. And finally, I show that the heart drug amiodarone, also known to have antifungal activity, blocks ergosterol biosynthesis at the level of oxidosqualene cyclase in Trypanosoma cruzi, work that has led to its use in the clinic as a novel antiparasitic agent. In each of these four examples, I use information from one area (organometallic chemistry, bone resorption drugs, cholesterol-lowering agents, heart disease) to develop drug leads in an unrelated area: a "knowledge-based" approach that represents an important advance in the search for new drugs.
Figures



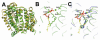

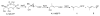





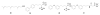
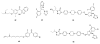
References
-
- Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep. 1999;16:565–74. - PubMed
-
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–30. - PubMed
-
- Seemann M, Bui BT, Wolff M, Tritsch D, Campos N, Boronat A, Marquet A, Rohmer M. Isoprenoid biosynthesis through the methylerythritol phosphate pathway: The (E)-4- hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE) is a [4Fe-4S] protein. Angew Chem Int Ed Engl. 2002;41:4337–9. - PubMed
-
- Wolff M, Seemann M, Tse Sum Bui B, Frapart Y, Tritsch D, Estrabot A. Garcia, Rodriguez-Concepcion M, Boronat A, Marquet A, Rohmer M. Isoprenoid biosynthesis via the methylerythritol phosphate pathway: The (E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase (LytB/IspH) from Escherichia coli is a [4Fe-4S] protein. FEBS Lett. 2003;541:115–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

