5alphaDH-DOC (5alpha-dihydro-deoxycorticosterone) activates androgen receptor in castration-resistant prostate cancer
- PMID: 20560974
- PMCID: PMC11158608
- DOI: 10.1111/j.1349-7006.2010.01620.x
5alphaDH-DOC (5alpha-dihydro-deoxycorticosterone) activates androgen receptor in castration-resistant prostate cancer
Abstract
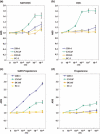
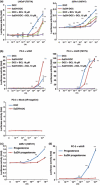
Prostate cancer often relapses during androgen-depletion therapy, even under the castration condition in which circulating androgens are drastically reduced. High expressions of androgen receptor (AR) and genes involved in androgen metabolism indicate a continued role for AR in castration-resistant prostate cancers (CRPCs). There is increasing evidence that some amounts of 5alpha-dihydrotestosterone (DHT) and other androgens are present sufficiently to activate AR within CRPC tissues, and enzymes involved in the androgen and steroid metabolism, such as 5alpha-steroid reductases, are activated in CRPCs. In this report, we screened eight natural 5alphaDH-steroids to search for novel products of 5alpha-steroid reductases, and identified 11-deoxycorticosterone (DOC) as a novel substrate for 5alpha-steroid reductases in CRPCs. 11-Deoxycorticosterone (DOC) and 5alpha-dihydro-deoxycorticosterone (5alphaDH-DOC) could promote prostate cancer cell proliferation through AR activation, and type 1 5alpha-steroid reductase (SRD5A1) could convert from DOC to 5alphaDH-DOC. Sensitive liquid chromatography-tandem mass spectrometric analysis detected 5alphaDH-DOC in some clinical CRPC tissues. These findings implicated that under an extremely low level of DHT, 5alphaDH-DOC and other products of 5alpha-steroid reductases within CRPC tissues might activate the AR pathway for prostate cancer cell proliferation and survival under castration.
Figures


Similar articles
-
Beyond T and DHT - novel steroid derivatives capable of wild type androgen receptor activation.Int J Biol Sci. 2014 Jun 3;10(6):602-13. doi: 10.7150/ijbs.8844. eCollection 2014. Int J Biol Sci. 2014. PMID: 24948873 Free PMC article. Review.
-
11β-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer?Mol Cell Endocrinol. 2013 Sep 5;377(1-2):135-46. doi: 10.1016/j.mce.2013.07.006. Epub 2013 Jul 13. Mol Cell Endocrinol. 2013. PMID: 23856005
-
Inverse Regulation of DHT Synthesis Enzymes 5α-Reductase Types 1 and 2 by the Androgen Receptor in Prostate Cancer.Endocrinology. 2017 Apr 1;158(4):1015-1021. doi: 10.1210/en.2016-1926. Endocrinology. 2017. PMID: 28324044
-
5α-reductase type 3 enzyme in benign and malignant prostate.Prostate. 2014 Feb;74(3):235-49. doi: 10.1002/pros.22745. Epub 2013 Oct 22. Prostate. 2014. PMID: 24150795 Free PMC article.
-
The 5α-androstanedione pathway to dihydrotestosterone in castration-resistant prostate cancer.J Investig Med. 2012 Feb;60(2):504-7. doi: 10.2310/JIM.0b013e31823874a4. J Investig Med. 2012. PMID: 22064602 Free PMC article. Review.
Cited by
-
Beyond T and DHT - novel steroid derivatives capable of wild type androgen receptor activation.Int J Biol Sci. 2014 Jun 3;10(6):602-13. doi: 10.7150/ijbs.8844. eCollection 2014. Int J Biol Sci. 2014. PMID: 24948873 Free PMC article. Review.
-
Molecular landscape for risk prediction and personalized therapeutics of castration-resistant prostate cancer: at a glance.Front Endocrinol (Lausanne). 2024 Jun 3;15:1360430. doi: 10.3389/fendo.2024.1360430. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38887275 Free PMC article. Review.
-
Relationship between SRD5A2 rs9282858 polymorphism and the susceptibility of prostate cancer: A meta-analysis based on 20 publications.Medicine (Baltimore). 2017 May;96(19):e6791. doi: 10.1097/MD.0000000000006791. Medicine (Baltimore). 2017. PMID: 28489754 Free PMC article.
References
-
- Gronberg H. Prostate cancer epidemiology. Lancet 2003; 361: 859–64. - PubMed
-
- Smaletz O, Scher HI. Outcome predictions for patients with metastatic prostate cancer. Semin Urol Oncol 2002; 20: 155–63. - PubMed
-
- Petrylak DP, Tangen CM, Hussain MHA et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–20. - PubMed
-
- Tannock IF, De Wit R, Berry WR et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–12. - PubMed
-
- Scher HI, Sawyers CL. Biology of progressive, castration‐resistant prostate cancer: directed therapies targeting the androgen‐receptor signaling axis. J Clin Oncol 2005; 23: 8253–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

