Medical device related pressure ulcers in hospitalized patients
- PMID: 20561094
- PMCID: PMC7951307
- DOI: 10.1111/j.1742-481X.2010.00699.x
Medical device related pressure ulcers in hospitalized patients
Abstract
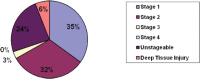
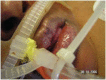
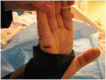
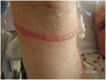
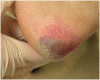
Most pressure ulcers occur over bony prominences such as heels and the sacrum. However, the National Pressure Ulcer Advisory Panel recognises that pressure ulcers can also occur on any tissue under pressure and thereby can develop beneath medical devices. This article reports on results from a secondary analysis of existing data collected by The Nebraska Medical Center on pressure ulcer quality improvement initiatives and outcomes. The purpose of this study was to quantify the extent of the problem and identify risk factors for medical device related (MDR) pressure ulcer development in hospitalised patients. A subset of data collected during eight quarterly pressure ulcer incidence and prevalence studies (N = 2178) was created and analysed. The overall rate of hospital-acquired pressure ulcers was 5·4% (113 of 2079). The proportion of patients with hospital-acquired ulcers related to medical devices was 34·5% (39 of 113). Findings indicate that if a patient had a medical device, they were 2·4 times more likely to develop a pressure ulcer of any kind. Numerous risk factors for pressure ulcer development were identified; however, none differentiated between those with MDR and traditional pressure ulcers.
© 2010 The Authors. Journal Compilation © 2010 Blackwell Publishing Ltd and Medicalhelplines.com Inc 2010.
Figures


References
-
- National Pressure Ulcer Advisory Panel (NPUAP) and European Pressure Ulcer Advisory Panel. International pressure ulcer prevention and treatment guidelines 2010 [WWW document]. URL http://www.npuap.org [accessed 1 April 2010].
-
- Reger S, Ranganathan V, Sahgal V. Support surface interface pressure, microclimate and the prevalence of pressure ulcers: an analysis of the literature. Ostomy Wound Manage 2007;53:50–8. - PubMed
-
- Wolverton CL, Hobbs LA, Beeson T, Benjamin M, Campbell K, Forbes C, Huff N, Kieninger M, Luebbehusen M, Myers M, White S. Nosocomial pressure ulcer rates in critical care [Electronic version]. J Nurs Care Qual 2004;20:56–62. - PubMed
-
- Davis JW, Parks SN, Detlefs CL, Williams GG, Williams JL, Smith RW. Clearing the cervical spine in obtunded patients: the use of dynamic fluoroscopy. J Trauma 1995;39:435–8. - PubMed
-
- Wille J, Braams R, Van Harren W, Van Der Werken C. Pulse oximeter‐induced digital injury: frequency rate and possible causative factors. Crit Care Med 2000;28:3555–7. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

