Cerebral venous sinus (sinovenous) thrombosis in children
- PMID: 20561500
- PMCID: PMC2892748
- DOI: 10.1016/j.nec.2010.03.006
Cerebral venous sinus (sinovenous) thrombosis in children
Abstract
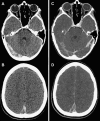
Cerebral venous sinus (sinovenous) thrombosis (CSVT) in childhood is a rare, but underrecognized, disorder, typically of multifactorial etiology, with neurologic sequelae apparent in up to 40% of survivors and mortality approaching 10%. There is an expanding spectrum of perinatal brain injury associated with neonatal CSVT. Although there is considerable overlap in risk factors for CSVT in neonates and older infants and children, specific differences exist between the groups. Clinical symptoms are frequently nonspecific, which may obscure the diagnosis and delay treatment. While morbidity and mortality are significant, CSVT recurs less commonly than arterial ischemic stroke in children. Appropriate management may reduce the risk of recurrence and improve outcome, however there are no randomized controlled trials to support the use of anticoagulation in children. Although commonly employed in many centers, this practice remains controversial, highlighting the continued need for high-quality studies. This article reviews the literature pertaining to pediatric venous sinus thrombosis.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Dural sinus thrombosis with marked enlargement of the venous sinus--case report.Neurol Med Chir (Tokyo). 2010 Jan;50(1):59-61. doi: 10.2176/nmc.50.59. Neurol Med Chir (Tokyo). 2010. PMID: 20098029
-
Clinical Profile and Long-Term Outcome in Neonatal Cerebral Sinus Venous Thrombosis.Pediatr Neurol. 2021 Aug;121:20-25. doi: 10.1016/j.pediatrneurol.2021.05.001. Epub 2021 May 15. Pediatr Neurol. 2021. PMID: 34126318
-
Impact of anticoagulation on the short-term outcome in a population of neonates with cerebral sinovenous thrombosis: a retrospective study.J Child Neurol. 2011 Jul;26(7):844-50. doi: 10.1177/0883073810395142. Epub 2011 May 6. J Child Neurol. 2011. PMID: 21551369
-
Role of surgery in cerebral venous sinus thrombosis.J Pak Med Assoc. 2006 Nov;56(11):543-7. J Pak Med Assoc. 2006. PMID: 17183988 Review.
-
Intracranial dural arterio-venous fistula.Pract Neurol. 2008 Dec;8(6):362-9. doi: 10.1136/jnnp.2008.162008. Pract Neurol. 2008. PMID: 19015296 Review.
Cited by
-
Cerebral Venous Sinus Thrombosis Related to Iron-Deficiency Anemia.Cureus. 2020 Jun 29;12(6):e8917. doi: 10.7759/cureus.8917. Cureus. 2020. PMID: 32760618 Free PMC article.
-
Neurovascular disorders in children: an updated practical guide.Transl Pediatr. 2021 Apr;10(4):1100-1116. doi: 10.21037/tp-20-205. Transl Pediatr. 2021. PMID: 34012858 Free PMC article. Review.
-
Utility of Hounsfield unit and hematocrit values in the diagnosis of acute venous sinus thrombosis in unenhanced brain CTs in the pediatric population.Pediatr Radiol. 2019 Feb;49(2):234-239. doi: 10.1007/s00247-018-4273-y. Epub 2018 Oct 16. Pediatr Radiol. 2019. PMID: 30327829
-
Delayed Onset Bilateral Papilledema in a Young Boy's Eyes after Trauma.Medicina (Kaunas). 2022 Jan 17;58(1):140. doi: 10.3390/medicina58010140. Medicina (Kaunas). 2022. PMID: 35056449 Free PMC article.
-
Combined use of Solitaire FR and Penumbra devices for endovascular treatment of cerebral venous sinus thrombosis in a child.BMJ Case Rep. 2014 Feb 19;2014:bcr2013011088. doi: 10.1136/bcr-2013-011088. BMJ Case Rep. 2014. PMID: 24554672 Free PMC article.
References
-
- Mallick A.A., Sharples P.M., Calvert S.E. Cerebral venous sinus thrombosis: a case series including thrombolysis. Arch Dis Child. 2009;94:790–794. - PubMed
-
- Viera JP, Luis C, Monteiro JP, et al. Cerebral sinovenous thrombosis in children: clinical presentation and extension, localization and recanalization of thrombosis. Eur J Paediatr Neurol; 2010;14:80–5. - PubMed
-
- Wasay M., Dai A.I., Ansari M. Cerebral venous sinus thrombosis in children: a multicenter cohort from the United States. J Child Neurol. 2008;23:26–31. - PubMed
-
- Heller C., Heinecke A., Junker R. Cerebral venous thrombosis in children: a multifactorial origin. Circulation. 2003;108:1362–1367. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

