Synthesis of an Fmoc-threonine bearing core-2 glycan: a building block for PSGL-1 via Fmoc-assisted solid-phase peptide synthesis
- PMID: 20561607
- PMCID: PMC2902660
- DOI: 10.1016/j.carres.2010.05.004
Synthesis of an Fmoc-threonine bearing core-2 glycan: a building block for PSGL-1 via Fmoc-assisted solid-phase peptide synthesis
Abstract
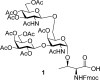
Selectins (L, E, and P) are vascular endothelial molecules that play an important role in the recruitment of leukocytes to inflamed tissue. In this regard, P-Selectin glycoprotein-1 (PSGL-1) has been identified as a ligand for P-Selectin. PSGL-1 binds to P-Selectin through the interaction of core-2 O-glycan expressing sialyl Lewis(x) oligosaccharide and the three tyrosine sulfate residues. Herein, we report the synthesis of threonine-linked core-2 O-glycan as an amino acid building block for the synthesis of PSGL-1. This building block was further incorporated in the Fmoc-assisted solid-phase peptide synthesis to provide a portion of the PSGL-1 glycopeptide.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Human L-selectin preferentially binds synthetic glycosulfopeptides modeled after endoglycan and containing tyrosine sulfate residues and sialyl Lewis x in core 2 O-glycans.Glycobiology. 2010 Sep;20(9):1170-85. doi: 10.1093/glycob/cwq083. Epub 2010 May 27. Glycobiology. 2010. PMID: 20507883 Free PMC article.
-
Rapid assembly of oligosaccharides: a highly convergent strategy for the assembly of a glycosylated amino acid derived from PSGL-1.J Org Chem. 2009 Aug 21;74(16):6064-71. doi: 10.1021/jo901135k. J Org Chem. 2009. PMID: 19606831 Free PMC article.
-
Model glycosulfopeptides from P-selectin glycoprotein ligand-1 require tyrosine sulfation and a core 2-branched O-glycan to bind to L-selectin.J Biol Chem. 2003 Jul 18;278(29):26391-400. doi: 10.1074/jbc.M303551200. Epub 2003 May 7. J Biol Chem. 2003. PMID: 12736247
-
Structure and function of the selectin ligand PSGL-1.Braz J Med Biol Res. 1999 May;32(5):519-28. doi: 10.1590/s0100-879x1999000500004. Braz J Med Biol Res. 1999. PMID: 10412562 Review.
-
PSGL-1 function in immunity and steady state homeostasis.Immunol Rev. 2009 Jul;230(1):75-96. doi: 10.1111/j.1600-065X.2009.00797.x. Immunol Rev. 2009. PMID: 19594630 Review.
Cited by
-
Automated Chemical Oligosaccharide Synthesis: Novel Approach to Traditional Challenges.Chem Rev. 2018 Sep 12;118(17):8105-8150. doi: 10.1021/acs.chemrev.8b00051. Epub 2018 Jun 28. Chem Rev. 2018. PMID: 29953217 Free PMC article. Review.
-
Glycopeptide analogues of PSGL-1 inhibit P-selectin in vitro and in vivo.Nat Commun. 2015 Mar 31;6:6387. doi: 10.1038/ncomms7387. Nat Commun. 2015. PMID: 25824568 Free PMC article.
-
Nanoparticles of a New Small-Molecule P-Selectin Inhibitor Attenuate Thrombosis, Inflammation, and Tumor Growth in Two Animal Models.Int J Nanomedicine. 2021 Aug 24;16:5777-5795. doi: 10.2147/IJN.S316863. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34471352 Free PMC article.
-
HPLC-Based Automated Synthesis of Glycans in Solution.Chemistry. 2022 Jul 11;28(39):e202201180. doi: 10.1002/chem.202201180. Epub 2022 May 25. Chemistry. 2022. PMID: 35513346 Free PMC article.
-
HPLC-assisted automated oligosaccharide synthesis: the implementation of the two-way split valve as a mode of complete automation.Chem Commun (Camb). 2020 Jan 28;56(9):1333-1336. doi: 10.1039/c9cc08876h. Epub 2020 Jan 13. Chem Commun (Camb). 2020. PMID: 31930269 Free PMC article.
References
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Nat. Rev. Immunol. 2007;7:678–689. - PubMed
-
- Vestweber D, Blanks JE. Physiol. Rev. 2009;79:181–213. - PubMed
-
- Welply JK, Keene JL, Schmuke JJ, Howard SC. Biochim. Biophys. Acta. 1994;1197:215–226. - PubMed
-
- Wilkins PP, Moore KL, McEver RP, Cummings RD. J. Biol. Chem. 1995;270:22677–22680. - PubMed
- DeLuca M, Dunlop LC, Andrews RK, Flannery JV, Jr., Ettling R, Cumming DA, Veldman GM, Berndt MC. J. Biol. Chem. 1995;270:26734–26737. - PubMed
- Sako D, Comess KM, Barone KM, Camphausen RT, Cumming DA, Shaw GD. Cell. 1995;83:323–331. - PubMed
- Pouyani T, Seed B. Cell. 1995;83:333–343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

