Biodegradable poly(ethylene glycol) hydrogels based on a self-elimination degradation mechanism
- PMID: 20561680
- PMCID: PMC2941153
- DOI: 10.1016/j.biomaterials.2010.05.021
Biodegradable poly(ethylene glycol) hydrogels based on a self-elimination degradation mechanism
Abstract
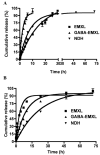
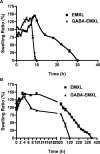
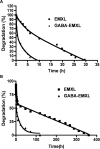
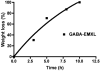
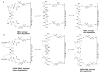
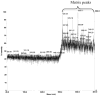
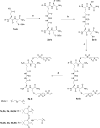
Two vinyl sulfone functionalized crosslinkers were developed for the purpose of preparing degradable poly(ethylene glycol) (PEG) hydrogels (EMXL and GABA-EMXL hydrogels). A self-elimination degradation mechanism in which an N-terminal residue of a glutamine is converted to pyroglutamic acid with subsequent release of diamino PEG (DAP) is proposed. The hydrogels were formed via Michael addition by mixing degradable or nondegradable crosslinkers and copolymer {4% w/v; poly[PEG-alt-poly(mercapto-succinic acid)]} at room temperature in phosphate buffer (PB, pH = 7.4). Hydrogel degradation was characterized by assessing diamino PEG release and examining morphological changes as well as the swelling and weight loss ratio under physiological conditions (37 degrees C). Degradation of EMXL and GABA-EMXL hydrogels occurred by surface erosion (confirmed by SEM). GABA-EMXL degradation was significantly faster (approximately 3-fold) than EMXL; however, the degradation of both hydrogels in mouse plasma was 12-times slower than in PBS. The slower degradation rate in plasma as compared to buffer is consistent with the presence of gamma-glutamyltransferase, gamma-glutamylcyclotransferase and/or glutaminyl cyclase (QC), which have been shown to suppress pyroglutamic acid formation. The current studies suggest that EMXL and GABA-EMXL hydrogels may have biomedical applications where 1-2 week degradation timeframes are optimal.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

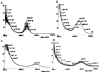




References
-
- Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–1879. - PubMed
-
- Fedorovich NE, Alblas J, de Wijn JR, Hennink WE, Verbout AJ, Dhert WJ. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng. 2007;13(8):1905–1925. - PubMed
-
- Lalloo A, Chao P, Hu P, Stein S, Sinko PJ. Pharmacokinetic and pharmacodynamic evaluation of a novel in situ forming poly(ethylene glycol)-based hydrogel for the controlled delivery of the camptothecins. J Control Release. 2006;112(3):333–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

