Applications of biological pores in nanomedicine, sensing, and nanoelectronics
- PMID: 20561776
- PMCID: PMC3121537
- DOI: 10.1016/j.copbio.2010.05.002
Applications of biological pores in nanomedicine, sensing, and nanoelectronics
Abstract
Biological protein pores and pore-forming peptides can generate a pathway for the flux of ions and other charged or polar molecules across cellular membranes. In nature, these nanopores have diverse and essential functions that range from maintaining cell homeostasis and participating in cell signaling to activating or killing cells. The combination of the nanoscale dimensions and sophisticated - often regulated - functionality of these biological pores make them particularly attractive for the growing field of nanobiotechnology. Applications range from single-molecule sensing to drug delivery and targeted killing of malignant cells. Potential future applications may include the use of nanopores for single strand DNA sequencing and for generating bio-inspired, and possibly, biocompatible visual detection systems and batteries. This article reviews the current state of applications of pore-forming peptides and proteins in nanomedicine, sensing, and nanoelectronics.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

















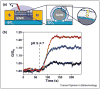

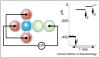
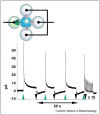
References
-
- Aidley DJ, Stanfield PR. Ion Channels: Molecules in Action. Cambridge: Cambridge University Press; 1996.
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4. New York: Garland Science; 2002.
-
- Bayley H, Cremer PS. Stochastic sensors inspired by biology. Nature. 2001;413:226–230. This article provides an insight to the application of engineered biological pores for development of rapid and sensitive biosensors with a focus on the application of these pores in stochastic sensing. - PubMed
-
- Panchal RG, Smart ML, Bowser DN, Williams DA, Petrou S. Pore-forming proteins and their application in biotechnology. Curr Pharm Biotechnol. 2002;3:99–115. This excellent review introduces a number of channel proteins and pore-forming peptides that have been used in biotechnology. It also provides a brief overview of the applications of these pores in medicine and sensing. - PubMed
-
- Estes DJ, Memarsadeghi S, Lundy SK, Marti F, Mikol DD, Fox DA, Mayer M. High-throughput profiling of ion channel activity in primary human lymphocytes. Anal Chem. 2008;80:3728–3735. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

