Chitin synthesis and fungal pathogenesis
- PMID: 20561815
- PMCID: PMC2923753
- DOI: 10.1016/j.mib.2010.05.002
Chitin synthesis and fungal pathogenesis
Abstract
Chitin is an essential part of the carbohydrate skeleton of the fungal cell wall and is a molecule that is not represented in humans and other vertebrates. Complex regulatory mechanisms enable chitin to be positioned at specific sites throughout the cell cycle to maintain the overall strength of the wall and enable rapid, life-saving modifications to be made under cell wall stress conditions. Chitin has also recently emerged as a significant player in the activation and attenuation of immune responses to fungi and other chitin-containing parasites. This review summarises latest advances in the analysis of chitin synthesis regulation in the context of fungal pathogenesis.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
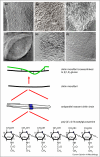
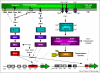
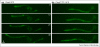
Comment in
-
Host-microbe interactions: fungi.Curr Opin Microbiol. 2010 Aug;13(4):389-91. doi: 10.1016/j.mib.2010.05.010. Epub 2010 Jun 16. Curr Opin Microbiol. 2010. PMID: 20558099 Free PMC article. No abstract available.
References
-
- Munro C.A., Gow N.A.R. Chitin synthesis in human pathogenic fungi. Med Mycol. 2001;39 S1:41–53. - PubMed
-
- Latge J.P. The cell wall: a carbohydrate armour for the fungal cell. Mol Microbiol. 2007;66:279–290. - PubMed
-
- Klis F.M., Boorsma A., De Groot P.W. Cell wall construction in Saccharomyces cerevisiae. Yeast. 2006;23:185–202. - PubMed
-
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

