Na/H exchanger regulatory factors control parathyroid hormone receptor signaling by facilitating differential activation of G(alpha) protein subunits
- PMID: 20562104
- PMCID: PMC2930697
- DOI: 10.1074/jbc.M110.147785
Na/H exchanger regulatory factors control parathyroid hormone receptor signaling by facilitating differential activation of G(alpha) protein subunits
Abstract
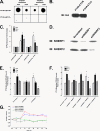
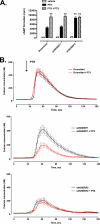
The Na/H exchanger regulatory factors, NHERF1 and NHERF2, are adapter proteins involved in targeting and assembly of protein complexes. The parathyroid hormone receptor (PTHR) interacts with both NHERF1 and NHERF2. The NHERF proteins toggle PTHR signaling from predominantly activation of adenylyl cyclase in the absence of NHERF to principally stimulation of phospholipase C when the NHERF proteins are expressed. We hypothesized that this signaling switch occurs at the level of the G protein. We measured G protein activation by [(35)S]GTPgammaS binding and G(alpha) subtype-specific immunoprecipitation using three different cellular models of PTHR signaling. These studies revealed that PTHR interactions with NHERF1 enhance receptor-mediated stimulation of G(alpha)(q) but have no effect on stimulation of G(alpha)(i) or G(alpha)(s). In contrast, PTHR associations with NHERF2 enhance receptor-mediated stimulation of both G(alpha)(q) and G(alpha)(i) but decrease stimulation of G(alpha)(s). Consistent with these functional data, NHERF2 formed cellular complexes with both G(alpha)(q) and G(alpha)(i), whereas NHERF1 was found to interact only with G(alpha)(q). These findings demonstrate that NHERF interactions regulate PTHR signaling at the level of G proteins and that NHERF1 and NHERF2 exhibit isotype-specific effects on G protein activation.
Figures
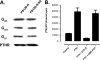
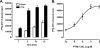
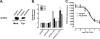

Similar articles
-
Formation of a ternary complex among NHERF1, beta-arrestin, and parathyroid hormone receptor.J Biol Chem. 2010 Sep 24;285(39):30355-62. doi: 10.1074/jbc.M110.114900. Epub 2010 Jul 23. J Biol Chem. 2010. PMID: 20656684 Free PMC article.
-
Dynamic Na+-H+ exchanger regulatory factor-1 association and dissociation regulate parathyroid hormone receptor trafficking at membrane microdomains.J Biol Chem. 2011 Oct 7;286(40):35020-9. doi: 10.1074/jbc.M111.264978. Epub 2011 Aug 8. J Biol Chem. 2011. PMID: 21832055 Free PMC article.
-
Origins of PDZ Binding Specificity. A Computational and Experimental Study Using NHERF1 and the Parathyroid Hormone Receptor.Biochemistry. 2017 May 23;56(20):2584-2593. doi: 10.1021/acs.biochem.7b00078. Epub 2017 Apr 14. Biochemistry. 2017. PMID: 28376304 Free PMC article.
-
Heterotrimeric G proteins in the control of parathyroid hormone actions.J Mol Endocrinol. 2017 May;58(4):R203-R224. doi: 10.1530/JME-16-0221. J Mol Endocrinol. 2017. PMID: 28363951 Free PMC article. Review.
-
From parathyroid hormone to cytosolic Ca2+ signals.Biochem Soc Trans. 2012 Feb;40(1):147-52. doi: 10.1042/BST20110615. Biochem Soc Trans. 2012. PMID: 22260681 Review.
Cited by
-
Cyclic AMP directs inositol (1,4,5)-trisphosphate-evoked Ca2+ signalling to different intracellular Ca2+ stores.J Cell Sci. 2013 May 15;126(Pt 10):2305-13. doi: 10.1242/jcs.126144. Epub 2013 Mar 22. J Cell Sci. 2013. PMID: 23525004 Free PMC article.
-
Disruption of β-catenin binding to parathyroid hormone (PTH) receptor inhibits PTH-stimulated ERK1/2 activation.Biochem Biophys Res Commun. 2015 Aug 14;464(1):27-32. doi: 10.1016/j.bbrc.2015.05.082. Epub 2015 Jun 3. Biochem Biophys Res Commun. 2015. PMID: 26047699 Free PMC article.
-
EBP50 inhibits the anti-mitogenic action of the parathyroid hormone type 1 receptor in vascular smooth muscle cells.J Mol Cell Cardiol. 2010 Dec;49(6):1012-21. doi: 10.1016/j.yjmcc.2010.08.025. Epub 2010 Sep 16. J Mol Cell Cardiol. 2010. PMID: 20843475 Free PMC article.
-
Ezrin-anchored protein kinase A coordinates phosphorylation-dependent disassembly of a NHERF1 ternary complex to regulate hormone-sensitive phosphate transport.J Biol Chem. 2012 Jul 13;287(29):24148-63. doi: 10.1074/jbc.M112.369405. Epub 2012 May 24. J Biol Chem. 2012. PMID: 22628548 Free PMC article.
-
GPCRs in the regulation of the functional activity of multipotent mesenchymal stromal cells.Front Cell Dev Biol. 2022 Aug 15;10:953374. doi: 10.3389/fcell.2022.953374. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36046341 Free PMC article. Review.
References
-
- Schwindinger W. F., Fredericks J., Watkins L., Robinson H., Bathon J. M., Pines M., Suva L. J., Levine M. A. (1998) Endocrine 8, 201–209 - PubMed
-
- Offermanns S., Iida-Klein A., Segre G. V., Simon M. I. (1996) Mol. Endocrinol. 10, 566–574 - PubMed
-
- Wu S., Pirola C. J., Green J., Yamaguchi D. T., Okano K., Jueppner H., Forrester J. S., Fagin J. A., Clemens T. L. (1993) Endocrinology 133, 2437–2444 - PubMed
-
- Maeda S., Wu S., Jüppner H., Green J., Aragay A. M., Fagin J. A., Clemens T. L. (1996) Endocrinology 137, 3154–3162 - PubMed
-
- Orloff J. J., Kats Y., Urena P., Schipani E., Vasavada R. C., Philbrick W. M., Behal A., Abou-Samra A. B., Segre G. V., Jüppner H. (1995) Endocrinology 136, 3016–3023 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

