Allosteric block of KCa2 channels by apamin
- PMID: 20562108
- PMCID: PMC2930706
- DOI: 10.1074/jbc.M110.110072
Allosteric block of KCa2 channels by apamin
Abstract
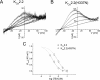
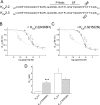
Activation of small conductance calcium-activated potassium (K(Ca)2) channels can regulate neuronal firing and synaptic plasticity. They are characterized by their high sensitivity to the bee venom toxin apamin, but the mechanism of block is not understood. For example, apamin binds to both K(Ca)2.2 and K(Ca)2.3 with the same high affinity (K(D) approximately 5 pM for both subtypes) but requires significantly higher concentrations to block functional current (IC(50) values of approximately 100 pM and approximately 5 nM, respectively). This suggests that steps beyond binding are needed for channel block to occur. We have combined patch clamp and binding experiments on cell lines with molecular modeling and mutagenesis to gain more insight into the mechanism of action of the toxin. An outer pore histidine residue common to both subtypes was found to be critical for both binding and block by the toxin but not for block by tetraethylammonium (TEA) ions. These data indicated that apamin blocks K(Ca)2 channels by binding to a site distinct from that used by TEA, supported by a finding that the onset of block by apamin was not affected by the presence of TEA. Structural modeling of ligand-channel interaction indicated that TEA binds deep within the channel pore, which contrasted with apamin being modeled to interact with the channel outer pore by utilizing the outer pore histidine residue. This multidisciplinary approach suggested that apamin does not behave as a classical pore blocker but blocks using an allosteric mechanism that is consistent with observed differences between binding affinity and potency of block.
Figures



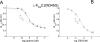


Similar articles
-
The interactions of apamin and tetraethylammonium are differentially affected by single mutations in the pore mouth of small conductance calcium-activated potassium (SK) channels.Biochem Pharmacol. 2013 Feb 15;85(4):560-9. doi: 10.1016/j.bcp.2012.12.015. Epub 2012 Dec 24. Biochem Pharmacol. 2013. PMID: 23270990
-
The relationship between functional inhibition and binding for K(Ca)2 channel blockers.PLoS One. 2013 Sep 10;8(9):e73328. doi: 10.1371/journal.pone.0073328. eCollection 2013. PLoS One. 2013. PMID: 24039913 Free PMC article.
-
Crucial role of a shared extracellular loop in apamin sensitivity and maintenance of pore shape of small-conductance calcium-activated potassium (SK) channels.Proc Natl Acad Sci U S A. 2011 Nov 8;108(45):18494-9. doi: 10.1073/pnas.1110724108. Epub 2011 Oct 24. Proc Natl Acad Sci U S A. 2011. PMID: 22025703 Free PMC article.
-
Ion-channel modulators: more diversity than previously thought.Chembiochem. 2011 Aug 16;12(12):1808-12. doi: 10.1002/cbic.201100236. Epub 2011 Jul 1. Chembiochem. 2011. PMID: 21726033 Review.
-
Small conductance calcium-activated potassium channels: from structure to function.Prog Neurobiol. 2010 Jul;91(3):242-55. doi: 10.1016/j.pneurobio.2010.03.002. Epub 2010 Mar 30. Prog Neurobiol. 2010. PMID: 20359520 Review.
Cited by
-
Novel Blocker of Onco SK3 Channels Derived from Scorpion Toxin Tamapin and Active against Migration of Cancer Cells.ACS Med Chem Lett. 2020 Jul 10;11(8):1627-1633. doi: 10.1021/acsmedchemlett.0c00300. eCollection 2020 Aug 13. ACS Med Chem Lett. 2020. PMID: 32832033 Free PMC article.
-
Inhibition of Small-Conductance, Ca2+-Activated K+ Current by Ondansetron.Front Pharmacol. 2021 Apr 22;12:651267. doi: 10.3389/fphar.2021.651267. eCollection 2021. Front Pharmacol. 2021. PMID: 33967791 Free PMC article.
-
International Union of Basic and Clinical Pharmacology. C. Nomenclature and Properties of Calcium-Activated and Sodium-Activated Potassium Channels.Pharmacol Rev. 2017 Jan;69(1):1-11. doi: 10.1124/pr.116.012864. Epub 2016 Nov 15. Pharmacol Rev. 2017. PMID: 28267675 Free PMC article. Review.
-
Hippocampal mGluR1-dependent long-term potentiation requires NAADP-mediated acidic store Ca2+ signaling.Sci Signal. 2018 Nov 27;11(558):eaat9093. doi: 10.1126/scisignal.aat9093. Sci Signal. 2018. PMID: 30482851 Free PMC article.
-
Endothelial small-conductance and intermediate-conductance KCa channels: an update on their pharmacology and usefulness as cardiovascular targets.J Cardiovasc Pharmacol. 2013 Feb;61(2):102-12. doi: 10.1097/FJC.0b013e318279ba20. J Cardiovasc Pharmacol. 2013. PMID: 23107876 Free PMC article. Review.
References
-
- Blatz A. L., Magleby K. L. (1986) Nature 323, 718–720 - PubMed
-
- Vincent J. P., Schweitz H., Lazdunski M. (1975) Biochemistry 14, 2521–2525 - PubMed
-
- Pease J. H., Wemmer D. E. (1988) Biochemistry 27, 8491–8498 - PubMed
-
- Labbé-Jullié C., Granier C., Albericio F., Defendini M. L., Ceard B., Rochat H., Van Rietschoten J. (1991) Eur. J. Biochem. 196, 639–645 - PubMed
-
- Köhler M., Hirschberg B., Bond C. T., Kinzie J. M., Marrion N. V., Maylie J., Adelman J. P. (1996) Science 273, 1709–1714 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

