Gravitropism of Arabidopsis thaliana roots requires the polarization of PIN2 toward the root tip in meristematic cortical cells
- PMID: 20562236
- PMCID: PMC2910985
- DOI: 10.1105/tpc.110.075317
Gravitropism of Arabidopsis thaliana roots requires the polarization of PIN2 toward the root tip in meristematic cortical cells
Abstract
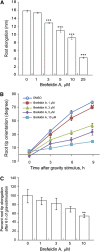
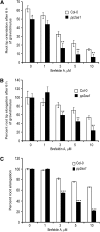
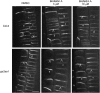
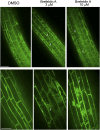
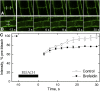
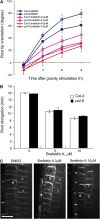
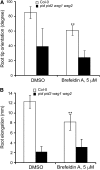
In the root, the transport of auxin from the tip to the elongation zone, referred to here as shootward, governs gravitropic bending. Shootward polar auxin transport, and hence gravitropism, depends on the polar deployment of the PIN-FORMED auxin efflux carrier PIN2. In Arabidopsis thaliana, PIN2 has the expected shootward localization in epidermis and lateral root cap; however, this carrier is localized toward the root tip (rootward) in cortical cells of the meristem, a deployment whose function is enigmatic. We use pharmacological and genetic tools to cause a shootward relocation of PIN2 in meristematic cortical cells without detectably altering PIN2 polarization in other cell types or PIN1 polarization. This relocation of cortical PIN2 was negatively regulated by the membrane trafficking factor GNOM and by the regulatory A1 subunit of type 2-A protein phosphatase (PP2AA1) but did not require the PINOID protein kinase. When GNOM was inhibited, PINOID abundance increased and PP2AA1 was partially immobilized, indicating both proteins are subject to GNOM-dependent regulation. Shootward PIN2 specifically in the cortex was accompanied by enhanced shootward polar auxin transport and by diminished gravitropism. These results demonstrate that auxin flow in the root cortex is important for optimal gravitropic response.
Figures






Similar articles
-
Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism.Nat Cell Biol. 2006 Mar;8(3):249-56. doi: 10.1038/ncb1369. Epub 2006 Feb 19. Nat Cell Biol. 2006. PMID: 16489343
-
Complex regulation of Arabidopsis AGR1/PIN2-mediated root gravitropic response and basipetal auxin transport by cantharidin-sensitive protein phosphatases.Plant J. 2005 Apr;42(2):188-200. doi: 10.1111/j.1365-313X.2005.02369.x. Plant J. 2005. PMID: 15807782
-
PINOID kinase regulates root gravitropism through modulation of PIN2-dependent basipetal auxin transport in Arabidopsis.Plant Physiol. 2009 Jun;150(2):722-35. doi: 10.1104/pp.108.131607. Epub 2009 Apr 10. Plant Physiol. 2009. PMID: 19363095 Free PMC article.
-
Auxin and Root Gravitropism: Addressing Basic Cellular Processes by Exploiting a Defined Growth Response.Int J Mol Sci. 2021 Mar 9;22(5):2749. doi: 10.3390/ijms22052749. Int J Mol Sci. 2021. PMID: 33803128 Free PMC article. Review.
-
Mechanisms of disruption of meristematic competence by microgravity in Arabidopsis seedlings.Plant Signal Behav. 2014;9(4):e28289. doi: 10.4161/psb.28289. Epub 2014 Mar 10. Plant Signal Behav. 2014. PMID: 24614101 Free PMC article. Review.
Cited by
-
Multiple roles for membrane-associated protein trafficking and signaling in gravitropism.Front Plant Sci. 2012 Dec 11;3:274. doi: 10.3389/fpls.2012.00274. eCollection 2012. Front Plant Sci. 2012. PMID: 23248632 Free PMC article.
-
Genome-Wide Analysis of the PIN Auxin Efflux Carrier Gene Family in Coffee.Plants (Basel). 2020 Aug 19;9(9):1061. doi: 10.3390/plants9091061. Plants (Basel). 2020. PMID: 32825074 Free PMC article.
-
Heterotrimeric G Protein-Regulated Ca2+ Influx and PIN2 Asymmetric Distribution Are Involved in Arabidopsis thaliana Roots' Avoidance Response to Extracellular ATP.Front Plant Sci. 2017 Sep 1;8:1522. doi: 10.3389/fpls.2017.01522. eCollection 2017. Front Plant Sci. 2017. PMID: 28919907 Free PMC article.
-
Gravistimulation effects on Oryza sativa amino acid profile, growth pattern and expression of OsPIN genes.Sci Rep. 2020 Oct 14;10(1):17303. doi: 10.1038/s41598-020-74531-w. Sci Rep. 2020. PMID: 33057095 Free PMC article.
-
Developmental Roles of AUX1/LAX Auxin Influx Carriers in Plants.Front Plant Sci. 2019 Oct 28;10:1306. doi: 10.3389/fpls.2019.01306. eCollection 2019. Front Plant Sci. 2019. PMID: 31719828 Free PMC article. Review.
References
-
- Baluška F., et al. (2005). What is apical and what is basal in plant root development? Trends Plant Sci. 10: 409–411 - PubMed
-
- Benjamins R., Malenica N., Luschnig C. (2005). Regulating the regulator: The control of auxin transport. Bioessays 27: 1246–1255 - PubMed
-
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 - PubMed
-
- Bennett S.R.M., Alvarez J., Bossinger G., Smyth D.R. (1995). Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J. 8: 505–520
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

