Transcriptomic insights into the physiology of Aspergillus niger approaching a specific growth rate of zero
- PMID: 20562270
- PMCID: PMC2918955
- DOI: 10.1128/AEM.00450-10
Transcriptomic insights into the physiology of Aspergillus niger approaching a specific growth rate of zero
Abstract
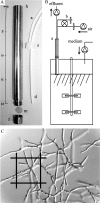
The physiology of filamentous fungi at growth rates approaching zero has been subject to limited study and exploitation. With the aim of uncoupling product formation from growth, we have revisited and improved the retentostat cultivation method for Aspergillus niger. A new retention device was designed allowing reliable and nearly complete cell retention even at high flow rates. Transcriptomic analysis was used to explore the potential for product formation at very low specific growth rates. The carbon- and energy-limited retentostat cultures were highly reproducible. While the specific growth rate approached zero (<0.005 h(-1)), the growth yield stabilized at a minimum (0.20 g of dry weight per g of maltose). The severe limitation led to asexual differentiation, and the supplied substrate was used for spore formation and secondary metabolism. Three physiologically distinct phases of the retentostat cultures were subjected to genome-wide transcriptomic analysis. The severe substrate limitation and sporulation were clearly reflected in the transcriptome. The transition from vegetative to reproductive growth was characterized by downregulation of genes encoding secreted substrate hydrolases and cell cycle genes and upregulation of many genes encoding secreted small cysteine-rich proteins and secondary metabolism genes. Transcription of known secretory pathway genes suggests that A. niger becomes adapted to secretion of small cysteine-rich proteins. The perspective is that A. niger cultures as they approach a zero growth rate can be used as a cell factory for production of secondary metabolites and cysteine-rich proteins. We propose that the improved retentostat method can be used in fundamental studies of differentiation and is applicable to filamentous fungi in general.
Figures

Similar articles
-
Submerged conidiation and product formation by Aspergillus niger at low specific growth rates are affected in aerial developmental mutants.Appl Environ Microbiol. 2011 Aug;77(15):5270-7. doi: 10.1128/AEM.00118-11. Epub 2011 Jun 7. Appl Environ Microbiol. 2011. PMID: 21652743 Free PMC article.
-
Transcriptomic comparison of Aspergillus niger growing on two different sugars reveals coordinated regulation of the secretory pathway.BMC Genomics. 2009 Jan 23;10:44. doi: 10.1186/1471-2164-10-44. BMC Genomics. 2009. PMID: 19166577 Free PMC article.
-
Highly active promoters and native secretion signals for protein production during extremely low growth rates in Aspergillus niger.Microb Cell Fact. 2016 Aug 20;15(1):145. doi: 10.1186/s12934-016-0543-2. Microb Cell Fact. 2016. PMID: 27544686 Free PMC article.
-
Physiological and Transcriptional Responses of Different Industrial Microbes at Near-Zero Specific Growth Rates.Appl Environ Microbiol. 2015 Sep 1;81(17):5662-70. doi: 10.1128/AEM.00944-15. Epub 2015 Jun 5. Appl Environ Microbiol. 2015. PMID: 26048933 Free PMC article. Review.
-
[Aspergillus niger as a potential cellular factory: prior knowledge and key technology].Sheng Wu Gong Cheng Xue Bao. 2010 Oct;26(10):1410-8. Sheng Wu Gong Cheng Xue Bao. 2010. PMID: 21218629 Review. Chinese.
Cited by
-
The low affinity glucose transporter HxtB is also involved in glucose signalling and metabolism in Aspergillus nidulans.Sci Rep. 2017 Mar 31;7:45073. doi: 10.1038/srep45073. Sci Rep. 2017. PMID: 28361917 Free PMC article.
-
Molecular and metabolic adaptations of Lactococcus lactis at near-zero growth rates.Appl Environ Microbiol. 2015 Jan;81(1):320-31. doi: 10.1128/AEM.02484-14. Epub 2014 Oct 24. Appl Environ Microbiol. 2015. PMID: 25344239 Free PMC article.
-
An Evolutionarily Conserved Transcriptional Activator-Repressor Module Controls Expression of Genes for D-Galacturonic Acid Utilization in Aspergillus niger.Genetics. 2017 Jan;205(1):169-183. doi: 10.1534/genetics.116.194050. Epub 2016 Nov 9. Genetics. 2017. PMID: 28049705 Free PMC article.
-
Current views on endocytosis in filamentous fungi.Mycology. 2020 Mar 24;12(1):1-9. doi: 10.1080/21501203.2020.1741471. Mycology. 2020. PMID: 33628604 Free PMC article.
-
Aspergillus as a multi-purpose cell factory: current status and perspectives.Biotechnol Lett. 2011 Mar;33(3):469-76. doi: 10.1007/s10529-010-0473-8. Epub 2010 Nov 19. Biotechnol Lett. 2011. PMID: 21088867 Free PMC article. Review.
References
-
- Affymetrix Inc. 2002. GeneChip Expression Analysis Technical Manual. Affymetrix, Santa Clara, CA.
-
- Andersen, M. R., and J. Nielsen. 2009. Current status of systems biology in aspergilli. Fungal Genet. Biol. 46:S180-190. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

