The LysR-type virulence activator AphB regulates the expression of genes in Vibrio cholerae in response to low pH and anaerobiosis
- PMID: 20562308
- PMCID: PMC2916415
- DOI: 10.1128/JB.00193-10
The LysR-type virulence activator AphB regulates the expression of genes in Vibrio cholerae in response to low pH and anaerobiosis
Abstract
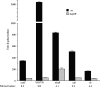
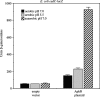
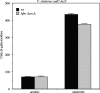
AphB is a LysR-type activator that initiates the expression of the virulence cascade in Vibrio cholerae by cooperating with the quorum-sensing-regulated activator AphA at the tcpPH promoter on the Vibrio pathogenicity island (VPI). To identify the ancestral chromosomal genes in V. cholerae regulated by AphB, we carried out a microarray analysis and show here that AphB influences the expression of a number of genes that are not associated with the VPI. One gene strongly activated by AphB is cadC, which encodes the ToxR-like transcriptional activator responsible for activating the expression of lysine decarboxylase, which plays an important role in survival at low pH. Other genes activated by AphB encode a Na(+)/H(+) antiporter, a carbonic anhydrase, a member of the ClC family of chloride channels, and a member of the Gpr1/Fun34/YaaH family. AphB influences each of these genes directly by recognizing a conserved binding site within their promoters, as determined by gel mobility shift assays. Transcriptional lacZ fusions indicate that AphB activates the expression of these genes under aerobic conditions in response to low pH and also under anaerobic conditions at neutral pH. Further experiments show that the regulation of cadC by AphB in response to low pH and anaerobiosis is mirrored in the heterologous organism Escherichia coli, is independent of the global regulators Fnr and ArcAB, and depends upon the region of the promoter that contains the AphB binding site. These results raise the possibility that the activity of AphB is influenced by the pH and oxygen tension of the environment.
Figures


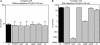

Similar articles
-
Dual regulation of genes involved in acetoin biosynthesis and motility/biofilm formation by the virulence activator AphA and the acetate-responsive LysR-type regulator AlsR in Vibrio cholerae.Mol Microbiol. 2005 Jul;57(2):420-33. doi: 10.1111/j.1365-2958.2005.04700.x. Mol Microbiol. 2005. PMID: 15978075
-
Overlapping binding sites for the virulence gene regulators AphA, AphB and cAMP-CRP at the Vibrio cholerae tcpPH promoter.Mol Microbiol. 2001 Jul;41(2):393-407. doi: 10.1046/j.1365-2958.2001.02518.x. Mol Microbiol. 2001. PMID: 11489126
-
AphB influences acid tolerance of Vibrio vulnificus by activating expression of the positive regulator CadC.J Bacteriol. 2006 Sep;188(18):6490-7. doi: 10.1128/JB.00533-06. J Bacteriol. 2006. PMID: 16952939 Free PMC article.
-
Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae.Mol Microbiol. 1992 Feb;6(4):451-8. doi: 10.1111/j.1365-2958.1992.tb01489.x. Mol Microbiol. 1992. PMID: 1560773 Review.
-
Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli.Mol Microbiol. 1997 Sep;25(6):1003-9. doi: 10.1046/j.1365-2958.1997.5481909.x. Mol Microbiol. 1997. PMID: 9350858 Review.
Cited by
-
Vibrio cholerae OmpR Represses the ToxR Regulon in Response to Membrane Intercalating Agents That Are Prevalent in the Human Gastrointestinal Tract.Infect Immun. 2020 Feb 20;88(3):e00912-19. doi: 10.1128/IAI.00912-19. Print 2020 Feb 20. Infect Immun. 2020. PMID: 31871096 Free PMC article.
-
Structural Insights into Regulation of Vibrio Virulence Gene Networks.Adv Exp Med Biol. 2023;1404:269-294. doi: 10.1007/978-3-031-22997-8_14. Adv Exp Med Biol. 2023. PMID: 36792881
-
Expression of the AHPND Toxins PirAvp and PirBvp Is Regulated by Components of the Vibrio parahaemolyticus Quorum Sensing (QS) System.Int J Mol Sci. 2022 Mar 7;23(5):2889. doi: 10.3390/ijms23052889. Int J Mol Sci. 2022. PMID: 35270031 Free PMC article.
-
Cholera toxin production during anaerobic trimethylamine N-oxide respiration is mediated by stringent response in Vibrio cholerae.J Biol Chem. 2014 May 9;289(19):13232-42. doi: 10.1074/jbc.M113.540088. Epub 2014 Mar 19. J Biol Chem. 2014. PMID: 24648517 Free PMC article.
-
Physiological, Structural, and Functional Analysis of the Paralogous Cation-Proton Antiporters of NhaP Type from Vibrio cholerae.Int J Mol Sci. 2019 May 25;20(10):2572. doi: 10.3390/ijms20102572. Int J Mol Sci. 2019. PMID: 31130620 Free PMC article. Review.
References
-
- Bauer, C. E., S. Elsen, and T. H. Bird. 1999. Mechanisms for redox control of gene expression. Annu. Rev. Microbiol. 53:495-523. - PubMed
-
- Carroll, P. A., K. T. Tashima, M. B. Rogers, V. J. DiRita, and S. B. Calderwood. 1997. Phase variation in tcpH modulates expression of the ToxR regulon in Vibrio cholerae. Mol. Microbiol. 25:1099-1111. - PubMed
-
- Champion, G. A., M. N. Neely, M. A. Brennan, and V. J. DiRita. 1997. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol. Microbiol. 23:323-331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

