Interaction between KIR3DS1 and HLA-Bw4 predicts for progression-free survival after autologous stem cell transplantation in patients with multiple myeloma
- PMID: 20562327
- PMCID: PMC6143153
- DOI: 10.1182/blood-2010-03-273706
Interaction between KIR3DS1 and HLA-Bw4 predicts for progression-free survival after autologous stem cell transplantation in patients with multiple myeloma
Abstract
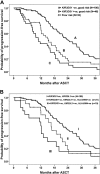
Natural killer (NK) cells exert antimyeloma cytotoxicity. The balance between inhibition and activation of NK-cells played by the inherited repertoire of killer immunoglobulin-like receptor (KIR) genes therefore may influence prognosis. One hundred eighty-two patients with multiple myeloma (MM) were analyzed for KIR repertoire. Multivariate analysis showed that progression-free survival (PFS) after autologous stem cell transplantation (ASCT) was significantly shorter for patients who are KIR3DS1(+) (P = .01). This was most evident for patients in complete or partial remission (good risk; GR) at ASCT. The relative risk (RR) of progression or death for patients with KIR3DS1(+) compared with KIR3DS1(-) was 1.9 (95% CI, 1.3-3.1; P = .002). The most significant difference in PFS was observed in patients with GR KIR3DS1(+) in whom HLA-Bw4, the ligand for the corresponding inhibitory receptor KIR3DL1, was missing. Patients with KIR3DS1(+) KIR3DL1(+) HLA-Bw4(-) had a significantly shorter PFS than patients who were KIR3DS1(-), translating to a difference in median PFS of 12 months (12.2 vs 24 months; P = .002). Our data show that KIR-human leukocyte antigen immunogenetics represent a novel prognostic tool for patients with myeloma, shown here in the context of ASCT, and that KIR3DS1 positivity may identify patients at greater risk of progression.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
References
-
- Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335(2):91–97. - PubMed
-
- Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883. - PubMed
-
- Palumbo A, Bringhen S, Petrucci MT, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood. 2004;104(10):3052–3057. - PubMed
-
- Cavo M, Zamagni E, Tosi P, et al. Superiority of thalidomide and dexamethasone over vincristine-doxorubicindexamethasone (VAD) as primary therapy in preparation for autologous transplantation for multiple myeloma. Blood. 2005;106(1):35–39. - PubMed
-
- Lokhorst HM, Schmidt-Wolf I, Sonneveld P, et al. Thalidomide in induction treatment increases the very good partial response rate before and after high-dose therapy in previously untreated multiple myeloma. Haematologica. 2008;93(1):124–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

