H2O2 alters rat cardiac sarcomere function and protein phosphorylation through redox signaling
- PMID: 20562337
- PMCID: PMC2944474
- DOI: 10.1152/ajpheart.00050.2010
H2O2 alters rat cardiac sarcomere function and protein phosphorylation through redox signaling
Abstract
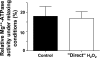
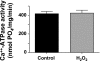
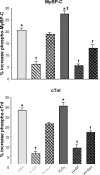
ROS, such as H(2)O(2), are a component of pathological conditions in many organ systems and have been reported to be elevated in cardiac pathophysiology. The experiments presented here test the hypothesis that H(2)O(2) induces alterations in cardiac myofilament function by the posttranslational modification of sarcomeric proteins indirectly through PKC signaling. In vitro assessment of actomyosin Mg(2+)-ATPase activity of myofibrillar fractions showed blunted relative ATP consumption in the relaxed state (pCa 8.0) in response to treatment with 0.5 mM H(2)O(2) before myofilament isolation. The effect was attributable to downstream "redox signaling," inasmuch as the direct application of H(2)O(2) to isolated myofibrils did not alter Mg(2+)-ATPase activity. Ca(2+)-ATPase activity, which was used as a measure of myofibrillar myosin function, was unaffected by H(2)O(2). Functional experiments using rat cardiac trabeculae treated with 0.5 or 5 mM H(2)O(2) followed by detergent extraction of membranes demonstrated increased Ca(2+) sensitivity of force production, a faster rate of force redevelopment, and (for 5 mM) decreased maximum tension. Biochemical analysis of myocardial samples treated with 0.5 mM H(2)O(2) demonstrated increased phosphorylation of two sarcomeric proteins: cardiac troponin I and myosin-binding protein-C. These changes were eliminated by a general PKC inhibitor. However, H(2)O(2) and the general PKC activator PMA induced different phosphorylation patterns in cardiomyocytes in which PKC-delta was elevated by viral infection. These data provide evidence that PKC-dependent redox signaling affects the function of cardiac myofilaments and indicate modification of specific proteins through this signaling mechanism.
Figures

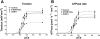


Similar articles
-
Relaxin alters cardiac myofilament function through a PKC-dependent pathway.Am J Physiol Heart Circ Physiol. 2009 Jul;297(1):H29-36. doi: 10.1152/ajpheart.00482.2008. Epub 2009 May 8. Am J Physiol Heart Circ Physiol. 2009. PMID: 19429819
-
Actin capping protein: an essential element in protein kinase signaling to the myofilaments.Circ Res. 2002 Jun 28;90(12):1299-306. doi: 10.1161/01.res.0000024389.03152.22. Circ Res. 2002. PMID: 12089068
-
Myosin heavy chain and cardiac troponin T damage is associated with impaired myofibrillar ATPase activity contributing to sarcomeric dysfunction in Ca2+-paradox rat hearts.Mol Cell Biochem. 2017 Jun;430(1-2):57-68. doi: 10.1007/s11010-017-2954-8. Epub 2017 Feb 17. Mol Cell Biochem. 2017. PMID: 28213770 Free PMC article.
-
Redox signaling and cardiac sarcomeres.J Biol Chem. 2011 Mar 25;286(12):9921-7. doi: 10.1074/jbc.R110.175489. Epub 2011 Jan 21. J Biol Chem. 2011. PMID: 21257753 Free PMC article. Review.
-
Oxidative stress and sarcomeric proteins.Circ Res. 2013 Jan 18;112(2):393-405. doi: 10.1161/CIRCRESAHA.111.300496. Circ Res. 2013. PMID: 23329794 Free PMC article. Review.
Cited by
-
Mitochondrial oxidative stress impairs contractile function but paradoxically increases muscle mass via fibre branching.J Cachexia Sarcopenia Muscle. 2019 Apr;10(2):411-428. doi: 10.1002/jcsm.12375. Epub 2019 Feb 1. J Cachexia Sarcopenia Muscle. 2019. PMID: 30706998 Free PMC article.
-
Impact of hydroxyl radical-induced injury on calcium handling and myofilament sensitivity in isolated myocardium.J Appl Physiol (1985). 2012 Sep 1;113(5):766-74. doi: 10.1152/japplphysiol.01424.2011. Epub 2012 Jul 5. J Appl Physiol (1985). 2012. PMID: 22773772 Free PMC article.
-
Mechanics on myocardium deficient in the N2B region of titin: the cardiac-unique spring element improves efficiency of the cardiac cycle.Biophys J. 2011 Sep 21;101(6):1385-92. doi: 10.1016/j.bpj.2011.06.054. Epub 2011 Sep 20. Biophys J. 2011. PMID: 21943419 Free PMC article.
-
Deliberate ROS production and auxin synergistically trigger the asymmetrical division generating the subsidiary cells in Zea mays stomatal complexes.Protoplasma. 2016 Jul;253(4):1081-99. doi: 10.1007/s00709-015-0866-6. Epub 2015 Aug 7. Protoplasma. 2016. PMID: 26250135
-
Mitochondrial Dysfunction in HFpEF: Potential Interventions Through Exercise.J Cardiovasc Transl Res. 2025 Apr;18(2):442-456. doi: 10.1007/s12265-025-10591-5. Epub 2025 Jan 25. J Cardiovasc Transl Res. 2025. PMID: 39863753 Review.
References
-
- Bayer AL, Heidkamp MC, Patel N, Porter M, Engman S, Samarel AM. Alterations in protein kinase C isoenzyme expression and autophosphorylation during the progression of pressure overload-induced left ventricular hypertrophy. Mol Cell Biochem 242: 145–152, 2003 - PubMed
-
- Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, de Tombe PP. Augmented protein kinase C-α-induced myofilament protein phosphorylation contrubutes to myofilament dysfunction in experimental congestive heart failure. Circ Res 101: 109–204, 2007 - PubMed
-
- Burkart EM, Sumandea MP, Kobayashi T, Nili M, Martin AF, Homsher E, Solaro RJ. Phosphorylation or glutamic acid substitution at protein kinase C sites on cardiac troponin I differentially depress myofilament tension and shortening velocity. J Biol Chem 278: 11265–11272, 2003 - PubMed
-
- Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal 8: 691–728, 2006 - PubMed
-
- Canton M, Neverova I, Menabo R, van Eyk J, di Lisa F. Evidence of myofibrillar protein oxidation induced by postischemic reperfusion in isolated rat hearts. Am J Physiol Heart Circ Physiol 286: H870–H877, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

