Self-organization of the phosphatidylinositol lipids signaling system for random cell migration
- PMID: 20562345
- PMCID: PMC2901478
- DOI: 10.1073/pnas.0908278107
Self-organization of the phosphatidylinositol lipids signaling system for random cell migration
Abstract
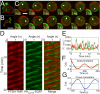
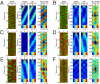
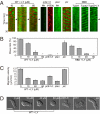
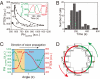
Phosphatidylinositol (PtdIns) lipids have been identified as key signaling mediators for random cell migration as well as chemoattractant-induced directional migration. However, how the PtdIns lipids are organized spatiotemporally to regulate cellular motility and polarity remains to be clarified. Here, we found that self-organized waves of PtdIns 3,4,5-trisphosphate [PtdIns(3,4,5)P(3)] are generated spontaneously on the membrane of Dictyostelium cells in the absence of a chemoattractant. Characteristic oscillatory dynamics within the PtdIns lipids signaling system were determined experimentally by observing the phenotypic variability of PtdIns lipid waves in single cells, which exhibited characteristics of a relaxation oscillator. The enzymes phosphatase and tensin homolog (PTEN) and phosphoinositide-3-kinase (PI3K), which are regulators for PtdIns lipid concentrations along the membrane, were essential for wave generation whereas functional actin cytoskeleton was not. Defects in these enzymes inhibited wave generation as well as actin-based polarization and cell migration. On the basis of these experimental results, we developed a reaction-diffusion model that reproduced the characteristic relaxation oscillation dynamics of the PtdIns lipid system, illustrating that a self-organization mechanism provides the basis for the PtdIns lipids signaling system to generate spontaneous spatiotemporal signals for random cell migration and that molecular noise derived from stochastic fluctuations within the signaling components gives rise to the variability of these spontaneous signals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

