EB66 cell line, a duck embryonic stem cell-derived substrate for the industrial production of therapeutic monoclonal antibodies with enhanced ADCC activity
- PMID: 20562528
- PMCID: PMC3180087
- DOI: 10.4161/mabs.12350
EB66 cell line, a duck embryonic stem cell-derived substrate for the industrial production of therapeutic monoclonal antibodies with enhanced ADCC activity
Abstract
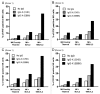
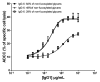
Monoclonal antibodies (mAbs) represent the fastest growing class of therapeutic proteins. The increasing demand for mAb manufacturing and the associated high production costs call for the pharmaceutical industry to improve its current production processes or develop more efficient alternative production platforms. The experimental control of IgG fucosylation to enhance antibody dependent cell cytotoxicity (ADCC) activity constitutes one of the promising strategies to improve the efficacy of monoclonal antibodies and to potentially reduce the therapeutic cost. We report here that the EB66 cell line derived from duck embryonic stem cells can be efficiently genetically engineered to produce mAbs at yields beyond a 1 g/L, as suspension cells grown in serum-free culture media. EB66 cells display additional attractive grown characteristics such as a very short population doubling time of 12 to 14 hours, a capacity to reach very high cell density (> 30 million cells/mL) and a unique metabolic profile resulting in low ammonium and lactate accumulation and low glutamine consumption, even at high cell densities. Furthermore, mAbs produced on EB66 cells display a naturally reduced fucose content resulting in strongly enhanced ADCC activity. The EB66 cells have therefore the potential to evolve as a novel cellular platform for the production of high potency therapeutic antibodies.
Figures
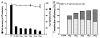
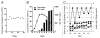
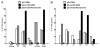

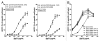
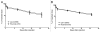
References
-
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22:1393–1398. - PubMed
-
- Butler M. Animal cell cultures: recent achievements and perspectives in the production of biopharmaceuticals. Appl Microbiol Biotechnol. 2005;68:283–291. - PubMed
-
- Rita Costa A, Elisa Rodrigues M, Henriques M, Azeredo J, Oliveira R. Guidelines to cell engineering for monoclonal antibody production. Eur J Pharm Biopharm. 2010;74:127–138. - PubMed
-
- Schirrmann T, Al-Halabi L, Dübel S, Hust M. Production systems for recombinant antibodies. Front Biosci. 2008;13:4576–4594. - PubMed
-
- Janeway CA, Travers P, Walport M, Shlomchik MJ, editors. Immunobiology, The immune system in health and disease. 5th edition. London: Garland Publishing; 2001. Antigen recognition by B cell and T cell receptors; pp. 93–122.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
