Cooperative interactions at the SLP-76 complex are critical for actin polymerization
- PMID: 20562827
- PMCID: PMC2910278
- DOI: 10.1038/emboj.2010.133
Cooperative interactions at the SLP-76 complex are critical for actin polymerization
Abstract
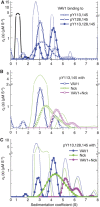
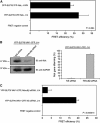
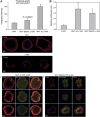
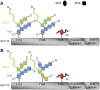
T-cell antigen receptor (TCR) engagement induces formation of multi-protein signalling complexes essential for regulating T-cell functions. Generation of a complex of SLP-76, Nck and VAV1 is crucial for regulation of the actin machinery. We define the composition, stoichiometry and specificity of interactions in the SLP-76, Nck and VAV1 complex. Our data reveal that this complex can contain one SLP-76 molecule, two Nck and two VAV1 molecules. A direct interaction between Nck and VAV1 is mediated by binding between the C-terminal SH3 domain of Nck and the VAV1 N-terminal SH3 domain. Disruption of the VAV1:Nck interaction deleteriously affected actin polymerization. These novel findings shed new light on the mechanism of actin polymerization after T-cell activation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Studying the dynamics of SLP-76, Nck, and Vav1 multimolecular complex formation in live human cells with triple-color FRET.Sci Signal. 2012 Apr 24;5(221):rs3. doi: 10.1126/scisignal.2002423. Sci Signal. 2012. PMID: 22534133
-
T cell specific adaptor protein (TSAd) promotes interaction of Nck with Lck and SLP-76 in T cells.Cell Commun Signal. 2015 Jul 11;13:31. doi: 10.1186/s12964-015-0109-7. Cell Commun Signal. 2015. PMID: 26163016 Free PMC article.
-
Studies of novel interactions between Nck and VAV SH3 domains.Commun Integr Biol. 2011 Mar;4(2):175-7. doi: 10.4161/cib.4.2.14235. Commun Integr Biol. 2011. PMID: 21655432 Free PMC article.
-
Integration of signaling and cytoskeletal remodeling by Nck in directional cell migration.Bioarchitecture. 2013 May-Jun;3(3):57-63. doi: 10.4161/bioa.25744. Epub 2013 Jul 17. Bioarchitecture. 2013. PMID: 23887203 Free PMC article. Review.
-
New insights into the T cell synapse from single molecule techniques.Nat Rev Immunol. 2011 Sep 9;11(10):672-84. doi: 10.1038/nri3066. Nat Rev Immunol. 2011. PMID: 21904389 Free PMC article. Review.
Cited by
-
Direct regulation of nephrin tyrosine phosphorylation by Nck adaptor proteins.J Biol Chem. 2013 Jan 18;288(3):1500-10. doi: 10.1074/jbc.M112.439463. Epub 2012 Nov 27. J Biol Chem. 2013. PMID: 23188823 Free PMC article.
-
Positive and negative regulation by SLP-76/ADAP and Pyk2 of chemokine-stimulated T-lymphocyte adhesion mediated by integrin α4β1.Mol Biol Cell. 2015 Sep 15;26(18):3215-28. doi: 10.1091/mbc.E14-07-1246. Epub 2015 Jul 22. Mol Biol Cell. 2015. PMID: 26202465 Free PMC article.
-
Functional cooperation between the proteins Nck and ADAP is fundamental for actin reorganization.Mol Cell Biol. 2011 Jul;31(13):2653-66. doi: 10.1128/MCB.01358-10. Epub 2011 May 2. Mol Cell Biol. 2011. PMID: 21536650 Free PMC article.
-
Functional nanoscale organization of signaling molecules downstream of the T cell antigen receptor.Immunity. 2011 Nov 23;35(5):705-20. doi: 10.1016/j.immuni.2011.10.004. Epub 2011 Nov 4. Immunity. 2011. PMID: 22055681 Free PMC article.
-
Monochromatic multicomponent fluorescence sedimentation velocity for the study of high-affinity protein interactions.Elife. 2016 Jul 20;5:e17812. doi: 10.7554/eLife.17812. Elife. 2016. PMID: 27436096 Free PMC article.
References
-
- Astoul E, Laurence AD, Totty N, Beer S, Alexander DR, Cantrell DA (2003) Approaches to define antigen receptor-induced serine kinase signal transduction pathways. J Biol Chem 278: 9267–9275 - PubMed
-
- Badour K, Zhang J, Shi F, McGavin MK, Rampersad V, Hardy LA, Field D, Siminovitch KA (2003) The Wiskott-Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity 18: 141–154 - PubMed
-
- Barda-Saad M, Braiman A, Titerence R, Bunnell SC, Barr VA, Samelson LE (2005) Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat Immunol 6: 80–89 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

