Stabilization of the spectrin-like domains of nesprin-1α by the evolutionarily conserved "adaptive" domain
- PMID: 20563238
- PMCID: PMC2885798
- DOI: 10.1007/s12195-010-0121-3
Stabilization of the spectrin-like domains of nesprin-1α by the evolutionarily conserved "adaptive" domain
Abstract
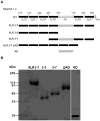
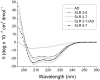
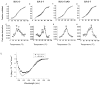
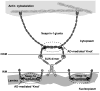
Nesprins are located at the outer and inner membranes of the nuclear envelope and help link the cytoskeleton to the nucleoskeleton. Nesprin-1α, located at the inner nuclear membrane, binds to A-type lamins and emerin and has homology to spectrin-repeat proteins. However, the mechanical and thermodynamic properties of the spectrin-like repeats (SLRs) of nesprin-1α and the potential structural contributions of the unique central domain were untested. In other spectrin superfamily proteins, tandem spectrin-repeat domains undergo cooperatively coupled folding and unfolding. We hypothesized that the large central domain, which interrupts SLRs and is conserved in other nesprin isoforms, might confer unique structural properties. To test this model we measured the thermal unfolding of nesprin-1α fragments using circular dichroism and dynamic light scattering. The SLRs in nesprin-1α were found to have structural and thermodynamic properties typical of spectrins. The central domain had relatively little secondary structure as an isolated fragment, but significantly stabilized larger SLR-containing molecules by increasing their overall helicity, thermal stability and cooperativity of folding. We suggest this domain, now termed the 'adaptive' domain (AD), also strengthens dimerization and inhibits unfolding. Further engineering of the isolated AD, and AD-containing nesprin molecules, may yield new information about the higher-order association of cooperative protein motifs.
Figures


Similar articles
-
Nesprin-1alpha self-associates and binds directly to emerin and lamin A in vitro.FEBS Lett. 2002 Aug 14;525(1-3):135-40. doi: 10.1016/s0014-5793(02)03105-8. FEBS Lett. 2002. PMID: 12163176
-
Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle.J Cell Sci. 2005 Feb 15;118(Pt 4):673-87. doi: 10.1242/jcs.01642. Epub 2005 Jan 25. J Cell Sci. 2005. PMID: 15671068
-
Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice.Hum Mol Genet. 2009 Feb 15;18(4):607-20. doi: 10.1093/hmg/ddn386. Epub 2008 Nov 13. Hum Mol Genet. 2009. PMID: 19008300 Free PMC article.
-
Nesprin-1: novel regulator of striated muscle nuclear positioning and mechanotransduction.Biochem Soc Trans. 2023 Jun 28;51(3):1331-1345. doi: 10.1042/BST20221541. Biochem Soc Trans. 2023. PMID: 37171063 Free PMC article. Review.
-
A Glance at the Nuclear Envelope Spectrin Repeat Protein 3.Biomed Res Int. 2019 Nov 20;2019:1651805. doi: 10.1155/2019/1651805. eCollection 2019. Biomed Res Int. 2019. PMID: 31828088 Free PMC article. Review.
Cited by
-
A Multisensory Network Drives Nuclear Mechanoadaptation.Biomolecules. 2022 Mar 4;12(3):404. doi: 10.3390/biom12030404. Biomolecules. 2022. PMID: 35327596 Free PMC article. Review.
-
Lamin A/C Is Required for ChAT-Dependent Neuroblastoma Differentiation.Mol Neurobiol. 2017 Jul;54(5):3729-3744. doi: 10.1007/s12035-016-9902-6. Epub 2016 May 25. Mol Neurobiol. 2017. PMID: 27221609
-
Beyond lamins other structural components of the nucleoskeleton.Methods Cell Biol. 2010;98:97-119. doi: 10.1016/S0091-679X(10)98005-9. Methods Cell Biol. 2010. PMID: 20816232 Free PMC article. Review.
-
Novel nesprin-1 mutations associated with dilated cardiomyopathy cause nuclear envelope disruption and defects in myogenesis.Hum Mol Genet. 2017 Jun 15;26(12):2258-2276. doi: 10.1093/hmg/ddx116. Hum Mol Genet. 2017. PMID: 28398466 Free PMC article.
-
Centrifugal Displacement of Nuclei Reveals Multiple LINC Complex Mechanisms for Homeostatic Nuclear Positioning.Curr Biol. 2017 Oct 23;27(20):3097-3110.e5. doi: 10.1016/j.cub.2017.08.073. Epub 2017 Oct 5. Curr Biol. 2017. PMID: 28988861 Free PMC article.
References
-
- Stewart CL, Roux KJ, Burke B. Blurring the boundary: the nuclear envelope extends its reach. Science. 2007;318:1408–1412. - PubMed
-
- Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat Rev Mol Cell Biol. 2005;6:21–31. - PubMed
-
- Zhang Q, Ragnauth CD, Skepper JN, Worth NF, Warren DT, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J Cell Sci. 2005;118:673–687. - PubMed
-
- Young KG, Kothary R. Spectrin repeat proteins in the nucleus. BioEssays. 2005;27:144–152. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

