Invasive glioblastoma cells acquire stemness and increased Akt activation
- PMID: 20563248
- PMCID: PMC2887086
- DOI: 10.1593/neo.10126
Invasive glioblastoma cells acquire stemness and increased Akt activation
Abstract
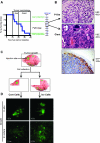
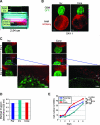
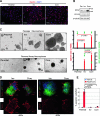
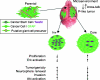
Glioblastoma multiforme (GBM) is the most frequent and most aggressive brain tumor in adults. The dismal prognosis is due to postsurgery recurrences arising from escaped invasive tumor cells. The signaling pathways activated in invasive cells are under investigation, and models are currently designed in search for therapeutic targets. We developed here an in vivo model of human invasive GBM in mouse brain from a GBM cell line with moderate tumorigenicity that allowed simultaneous primary tumor growth and dispersal of tumor cells in the brain parenchyma. This strategy allowed for the first time the isolation and characterization of matched sets of tumor mass (Core) and invasive (Inv) cells. Both cell populations, but more markedly Inv cells, acquired stem cell markers, neurosphere renewal ability, and resistance to rapamycin-induced apoptosis relative to parental cells. The comparative phenotypic analysis between Inv and Core cells showed significantly increased tumorigenicity in vivo and increased invasion with decreased proliferation in vitro for Inv cells. Examination of a large array of signaling pathways revealed extracellular signal-regulated kinase (Erk) down-modulation and Akt activation in Inv cells and an opposite profile in Core cells. Akt activation correlated with the increased tumorigenicity, stemness, and invasiveness, whereas Erk activation correlated with the proliferation of the cells. These results underscore complementary roles of the Erk and Akt pathways for GBM proliferation and dispersal and raise important implications for a concurrent inhibitory therapy.
Figures


Similar articles
-
Tumor microenvironment tenascin-C promotes glioblastoma invasion and negatively regulates tumor proliferation.Neuro Oncol. 2016 Apr;18(4):507-17. doi: 10.1093/neuonc/nov171. Epub 2015 Aug 27. Neuro Oncol. 2016. PMID: 26320116 Free PMC article.
-
Girdin maintains the stemness of glioblastoma stem cells.Oncogene. 2012 May 31;31(22):2715-24. doi: 10.1038/onc.2011.466. Epub 2011 Oct 24. Oncogene. 2012. PMID: 22020337
-
FoxO3a functions as a key integrator of cellular signals that control glioblastoma stem-like cell differentiation and tumorigenicity.Stem Cells. 2011 Sep;29(9):1327-37. doi: 10.1002/stem.696. Stem Cells. 2011. PMID: 21793107
-
Resveratrol as an antitumor agent for glioblastoma multiforme: Targeting resistance and promoting apoptotic cell deaths.Acta Histochem. 2023 Aug;125(6):152058. doi: 10.1016/j.acthis.2023.152058. Epub 2023 Jun 17. Acta Histochem. 2023. PMID: 37336070 Review.
-
Cancer stem cell contribution to glioblastoma invasiveness.Stem Cell Res Ther. 2013 Feb 28;4(1):18. doi: 10.1186/scrt166. Stem Cell Res Ther. 2013. PMID: 23510696 Free PMC article. Review.
Cited by
-
Overcoming intratumor heterogeneity of polygenic cancer drug resistance with improved biomarker integration.Neoplasia. 2012 Dec;14(12):1278-89. doi: 10.1593/neo.122096. Neoplasia. 2012. PMID: 23308059 Free PMC article.
-
Fibronectin Type III Domain Containing 3B as a Potential Prognostic and Therapeutic Biomarker for Glioblastoma.Biomedicines. 2023 Nov 28;11(12):3168. doi: 10.3390/biomedicines11123168. Biomedicines. 2023. PMID: 38137388 Free PMC article.
-
Three-dimensional (3D) tumor spheroid invasion assay.J Vis Exp. 2015 May 1;(99):e52686. doi: 10.3791/52686. J Vis Exp. 2015. PMID: 25993495 Free PMC article.
-
Exploiting metabolic differences in glioma therapy.Curr Drug Discov Technol. 2012 Dec;9(4):280-93. doi: 10.2174/157016312803305906. Curr Drug Discov Technol. 2012. PMID: 22339075 Free PMC article. Review.
-
PIM1 Inhibition Affects Glioblastoma Stem Cell Behavior and Kills Glioblastoma Stem-like Cells.Int J Mol Sci. 2021 Oct 15;22(20):11126. doi: 10.3390/ijms222011126. Int J Mol Sci. 2021. PMID: 34681783 Free PMC article.
References
-
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
