Teriflunomide (leflunomide) promotes cytostatic, antioxidant, and apoptotic effects in transformed prostate epithelial cells: evidence supporting a role for teriflunomide in prostate cancer chemoprevention
- PMID: 20563249
- PMCID: PMC2887087
- DOI: 10.1593/neo.10168
Teriflunomide (leflunomide) promotes cytostatic, antioxidant, and apoptotic effects in transformed prostate epithelial cells: evidence supporting a role for teriflunomide in prostate cancer chemoprevention
Abstract
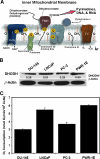
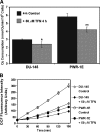
Teriflunomide (TFN) is an inhibitor of de novo pyrimidine synthesis and the active metabolite of leflunomide. Leflunomide is prescribed to patients worldwide as an immunomodulatory and anti-inflammatory disease-modifying prodrug. Leflunomide inhibited the growth of human prostate cancer xenographs in mice, and leflunomide or TFN promoted cytostasis and/or apoptosis in cultured cells. These findings suggest that TFN could be useful in prostate cancer chemoprevention. We investigated the possible mechanistic aspects of this tenet by characterizing the effects of TFN using premalignant PWR-1E and malignant DU-145 human prostate epithelial cells. TFN promoted a dose- and time-dependent cytostasis or apoptosis induction in these cells. The cytostatic effects of TFN, which were reversible but not by the presence of excess uridine in the culture medium, included diminished cellular uridine levels, an inhibition in oxygen consumption, a suppression of reactive oxygen species (ROS) generation, S-phase cell cycle arrest, and a conspicuous reduction in the size and number of the nucleoli in the nuclei of these cells. Conversely, TFN's apoptogenic effects were characteristic of catastrophic mitochondrial disruption (i.e., a dissipation of mitochondrial inner transmembrane potential, enhanced ROS production, mitochondrial cytochrome c release, and cytoplasmic vacuolization) and followed by DNA fragmentation. The respiration-deficient derivatives of the DU-145 cells, which are also uridine auxotrophs, were markedly resistant to the cytostatic and apoptotic effects of TFN, implicating de novo pyrimidine synthesis and mitochondrial bioenergetics as the primary targets for TFN in the respiration competent cells. These mechanistic findings advocate a role for TFN and mitochondrial bioenergetics in prostate cancer chemoprevention.
Figures






References
-
- Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. - PubMed
-
- Sun SY, Hail N, Jr, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst. 2004;96:662–672. - PubMed
-
- Evans DR, Guy HI. Mammalian pyrimidine biosynthesis: fresh insights into an ancient pathway. J Biol Chem. 2004;279:33035–33038. - PubMed
-
- Davis JP, Cain GA, Pitts WJ, Magolda RL, Copeland RA. The immunosuppressive metabolite of leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase. Biochemistry. 1996;35:1270–1273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
