Electrochemistry of redox-active self-assembled monolayers
- PMID: 20563297
- PMCID: PMC2885823
- DOI: 10.1016/j.ccr.2009.12.023
Electrochemistry of redox-active self-assembled monolayers
Abstract
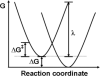
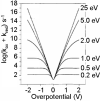
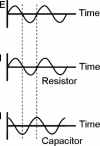
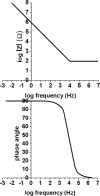
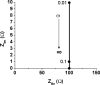
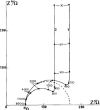
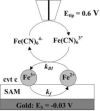
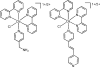
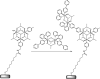
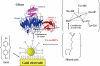
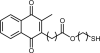
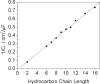
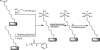
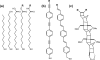
Redox-active self-assembled monolayers (SAMs) provide an excellent platform for investigating electron transfer kinetics. Using a well-defined bridge, a redox center can be positioned at a fixed distance from the electrode and electron transfer kinetics probed using a variety of electrochemical techniques. Cyclic voltammetry, AC voltammetry, electrochemical impedance spectroscopy, and chronoamperometry are most commonly used to determine the rate of electron transfer of redox-activated SAMs. A variety of redox species have been attached to SAMs, and include transition metal complexes (e.g., ferrocene, ruthenium pentaammine, osmium bisbipyridine, metal clusters) and organic molecules (e.g., galvinol, C(60)). SAMs offer an ideal environment to study the outer-sphere interactions of redox species. The composition and integrity of the monolayer and the electrode material influence the electron transfer kinetics and can be investigated using electrochemical methods. Theoretical models have been developed for investigating SAM structure. This review discusses methods and monolayer compositions for electrochemical measurements of redox-active SAMs.
Figures







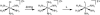

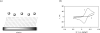
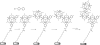










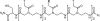

References
-
- Chidsey CED. Science. 1991;251:919. - PubMed
-
- Buriak JM. Chem. Commun. 1999:1051.
-
- Creager S, Yu CJ, Bamdad C, O'Connor S, MacLean T, Lam E, Chong Y, Olsen GT, Luo J, Gozin M, Kayyem JF. J. Am. Chem. Soc. 1999;121:1059.
-
- Diederich F, Gomez-Lopez M. Chem. Soc. Rev. 1999;28:263.
-
- Willner I, Katz E. Angew. Chem., Int. Ed. Engl. 2000;39:1181. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
