Coupling of a core post-translational pacemaker to a slave transcription/translation feedback loop in a circadian system
- PMID: 20563306
- PMCID: PMC2885980
- DOI: 10.1371/journal.pbio.1000394
Coupling of a core post-translational pacemaker to a slave transcription/translation feedback loop in a circadian system
Abstract
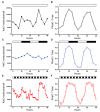
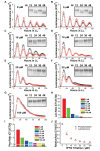
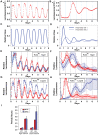
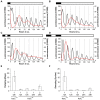
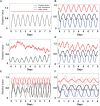
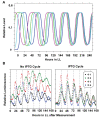
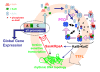
Cyanobacteria are the only model circadian clock system in which a circadian oscillator can be reconstituted in vitro. The underlying circadian mechanism appears to comprise two subcomponents: a post-translational oscillator (PTO) and a transcriptional/translational feedback loop (TTFL). The PTO and TTFL have been hypothesized to operate as dual oscillator systems in cyanobacteria. However, we find that they have a definite hierarchical interdependency-the PTO is the core pacemaker while the TTFL is a slave oscillator that quickly damps when the PTO stops. By analysis of overexpression experiments and mutant clock proteins, we find that the circadian system is dependent upon the PTO and that suppression of the PTO leads to damped TTFL-based oscillations whose temperature compensation is not stable under different metabolic conditions. Mathematical modeling indicates that the experimental data are compatible with a core PTO driving the TTFL; the combined PTO/TTFL system is resilient to noise. Moreover, the modeling indicates a mechanism by which the TTFL can feed into the PTO such that new synthesis of clock proteins can phase-shift or entrain the core PTO pacemaker. This prediction was experimentally tested and confirmed by entraining the in vivo circadian system with cycles of new clock protein synthesis that modulate the phosphorylation status of the clock proteins in the PTO. In cyanobacteria, the PTO is the self-sustained core pacemaker that can operate independently of the TTFL, but the TTFL damps when the phosphorylation status of the PTO is clamped. However, the TTFL can provide entraining input into the PTO. This study is the first to our knowledge to experimentally and theoretically investigate the dynamics of a circadian clock in which a PTO is coupled to a TTFL. These results have important implications for eukaryotic clock systems in that they can explain how a TTFL could appear to be a core circadian clockwork when in fact the true pacemaker is an embedded biochemical oscillator.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Attenuation of the posttranslational oscillator via transcription-translation feedback enhances circadian-phase shifts in Synechococcus.Proc Natl Acad Sci U S A. 2013 Aug 27;110(35):14486-91. doi: 10.1073/pnas.1302243110. Epub 2013 Aug 12. Proc Natl Acad Sci U S A. 2013. PMID: 23940358 Free PMC article.
-
Perfecting the Life Clock: The Journey from PTO to TTFL.Int J Mol Sci. 2023 Jan 26;24(3):2402. doi: 10.3390/ijms24032402. Int J Mol Sci. 2023. PMID: 36768725 Free PMC article. Review.
-
Circadian clocks and cell division: what's the pacemaker?Cell Cycle. 2010 Oct 1;9(19):3864-73. doi: 10.4161/cc.9.19.13205. Epub 2010 Oct 1. Cell Cycle. 2010. PMID: 20890114 Free PMC article.
-
Revealing a two-loop transcriptional feedback mechanism in the cyanobacterial circadian clock.PLoS Comput Biol. 2013;9(3):e1002966. doi: 10.1371/journal.pcbi.1002966. Epub 2013 Mar 14. PLoS Comput Biol. 2013. PMID: 23516349 Free PMC article.
-
A cyanobacterial circadian clockwork.Curr Biol. 2008 Sep 9;18(17):R816-R825. doi: 10.1016/j.cub.2008.07.012. Curr Biol. 2008. PMID: 18786387 Free PMC article. Review.
Cited by
-
PER2 Differentially Regulates Clock Phosphorylation versus Transcription by Reciprocal Switching of CK1ε Activity.J Biol Rhythms. 2015 Jun;30(3):206-16. doi: 10.1177/0748730415582127. J Biol Rhythms. 2015. PMID: 25994100 Free PMC article.
-
Circadian transcriptional regulation by the posttranslational oscillator without de novo clock gene expression in Synechococcus.Proc Natl Acad Sci U S A. 2011 Sep 13;108(37):15396-401. doi: 10.1073/pnas.1019612108. Epub 2011 Sep 6. Proc Natl Acad Sci U S A. 2011. PMID: 21896749 Free PMC article.
-
Attenuation of the posttranslational oscillator via transcription-translation feedback enhances circadian-phase shifts in Synechococcus.Proc Natl Acad Sci U S A. 2013 Aug 27;110(35):14486-91. doi: 10.1073/pnas.1302243110. Epub 2013 Aug 12. Proc Natl Acad Sci U S A. 2013. PMID: 23940358 Free PMC article.
-
Circadian clock component PERIOD2 regulates diurnal expression of Na+/H+ exchanger regulatory factor-1 and its scaffolding function.Sci Rep. 2018 Jun 13;8(1):9072. doi: 10.1038/s41598-018-27280-w. Sci Rep. 2018. PMID: 29899468 Free PMC article.
-
Does the precision of a biological clock depend upon its period? Effects of the duper and tau mutations in Syrian hamsters.PLoS One. 2012;7(5):e36119. doi: 10.1371/journal.pone.0036119. Epub 2012 May 15. PLoS One. 2012. PMID: 22615753 Free PMC article.
References
-
- Hardin P. E, Hall J. C, Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990;343:536–540. - PubMed
-
- Dunlap J. C, Loros J. J, DeCoursey P. J, editors. Chronobiology: biological timekeeping. 2004. Sinauer, Sunderland, MA.
-
- Ishiura M, Kutsuna S, Aoki S, Iwasaki H, Andersson C. R, et al. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science. 1998;281:1519–1523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

