Structural insights into G-quadruplexes: towards new anticancer drugs
- PMID: 20563318
- PMCID: PMC2886307
- DOI: 10.4155/fmc.09.172
Structural insights into G-quadruplexes: towards new anticancer drugs
Abstract
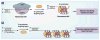
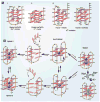
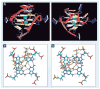
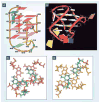
DNA G-quadruplexes are DNA secondary structures formed in specific G-rich sequences. DNA sequences that can form G-quadruplexes have been found in regions with biological significance, such as human telomeres and oncogene-promoter regions. DNA G-quadruplexes have recently emerged as a new class of novel molecular targets for anticancer drugs. Recent progress on structural studies of the biologically relevant G-quadruplexes formed in human telomeres and in the promoter regions of human oncogenes will be discussed, as well as recent advances in the design and development of G-quadruplex-interactive drugs. DNA G-quadruplexes can readily form in solution under physiological conditions and are globularly folded nucleic acid structures. The molecular structures of intramolecular G-quadruplexes appear to differ from one another and, therefore, in principle may be differentially regulated and targeted by different proteins and drugs.
Figures









References
-
- Sen D, Gilbert W. A sodium-potassium switch in the formation of four-stranded G4-DNA. 1990;344(6265):410–414. - PubMed
-
- Hud NV, Plavec J. The role of cations in determining quadruplex structure and stability. In: Neidle S, editor. Quadruplex Nucleic Acids. Royal Society of Chemistry Publishing; Cambridge UK: 2006. pp. 100–130.
-
- Neidle S, Parkinson G. Telomere maintenance as a target for anticancer drug discovery. Nat Rev Drug Discov. 2002;1(5):383–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
